Figures & data
Figure 1. Sketch of experimental device: 1, high-purity nitrogen; 2, sulfur dioxide; 3, nitrogen oxide; 4, oxygen; 5, mixing tank; 6, flow meter; 7, valve; 8 and 11, anti-leaking belt of microwave irradiation; 9, quartz tube; 10, activated carbon; 12, circulation pipe; 13, temperature display; 14, power adjustment button; 15, electric supply control system; 16, flue gas analyzer; 17, tail gas adsorption bottle; 18, emission; and 19, evaporation device.
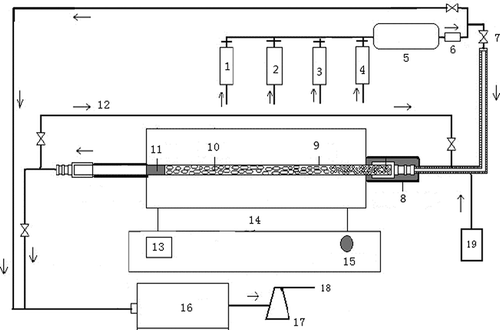
Table 1. Characteristics of fresh particle activated carbon used in this study
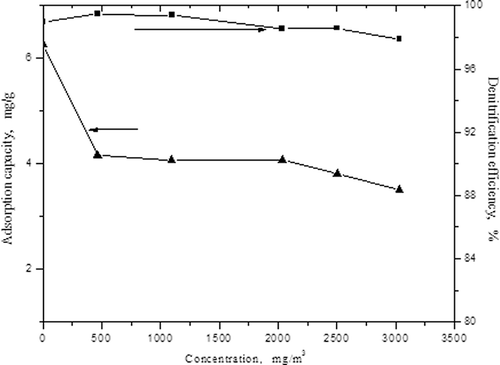
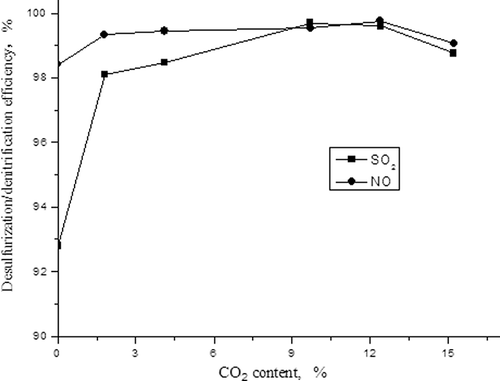
Figure 3. The influences of the concentrations of SO2 on adsorption capacity of NO and denitrification efficiency.