Figures & data
Figure 1. Effect of calcination temperature on surface area, pore volume, and pore diameter of the Mn/TiO2 catalysts.
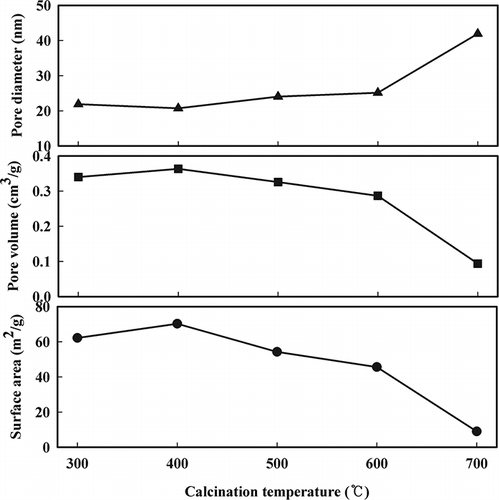
Figure 2. X-ray diffraction patterns of the Mn/TiO2 catalysts with different calcination temperatures (A = anatase TiO2; R = rutile TiO2).
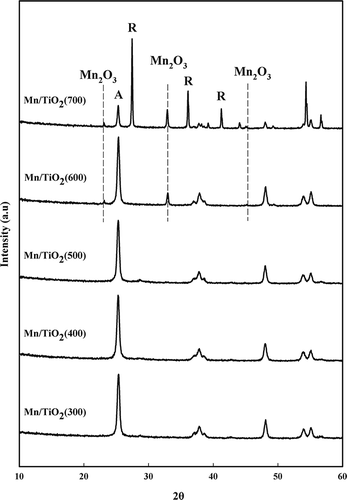
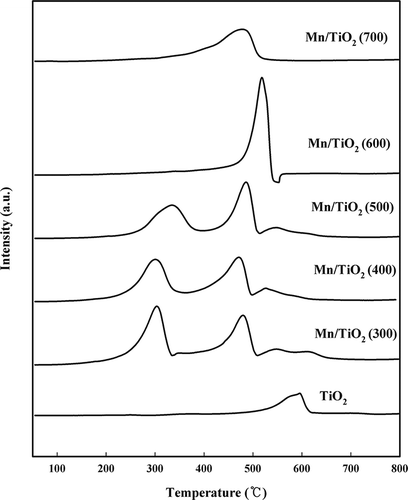
Figure 4. NH3-TPD profiles of the Mn/TiO2 catalysts with different calcination temperatures using TCD.
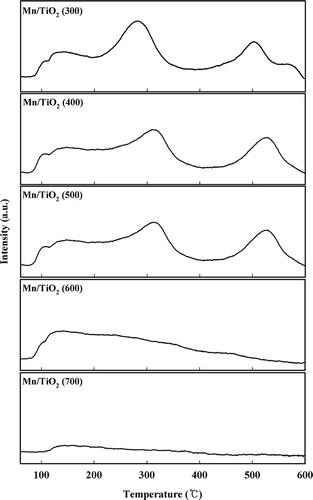
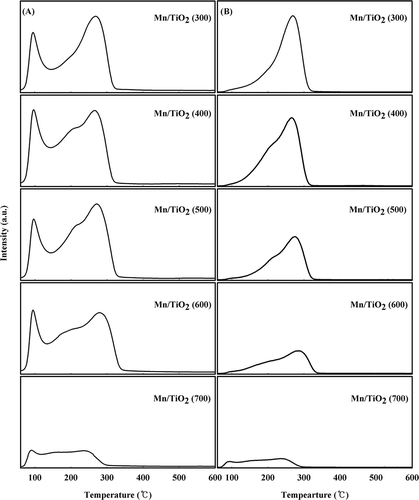
Figure 5. NH3-TPD profiles of the Mn/TiO2 catalysts with different calcination temperatures using mass spectrum. (A) Investigation of NH3, O2 and H2O; (B) investigation of N2, NO, N2O, and NO2 (color figure available online).
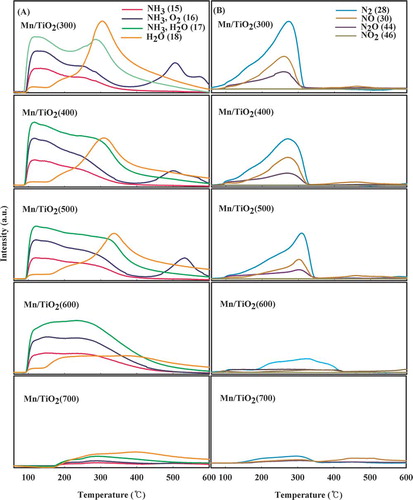
Figure 6. NO-TPD spectra of the Mn/TiO2 catalysts with different calcination temperatures using mass spectrum. (A) Investigation of NO; (B) investigation of NO2.