Figures & data
Table 1. XRF analysis
Table 2. Physical properties characterized by BET analysis
Table 3. Elemental analysis of Q-MCM, A-Q-MCM, and C-MCM
Figure 4. Recycle runs of CO2 by A-Q-MCM (adsorption at 25 °C and desorption at 100 °C for 120 min).
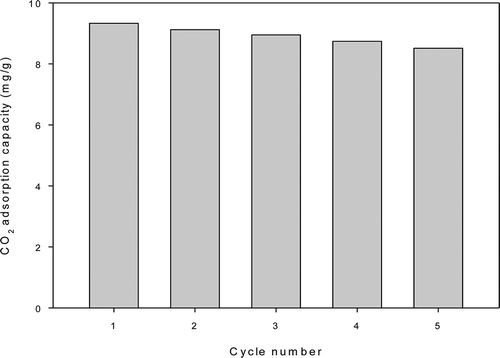
Table 4. CO2 adsorption properties of various microporous and mesoporous materials
Table 5. Summary of the Langmuir and Freundlich isotherm constants