Figures & data
Figure 1. Experimental system of fluidized-bed incinerator and activated carbon fibers (ACFs) adsorber: (1) air compressor; (2) flowmeter; (3) combustion chamber; (4) electrical heater; (5) thermal feedback controller; (6) thermocouple; (7) feeder; (8) ACF adsorber; (9) sampling; (10) induced fan.
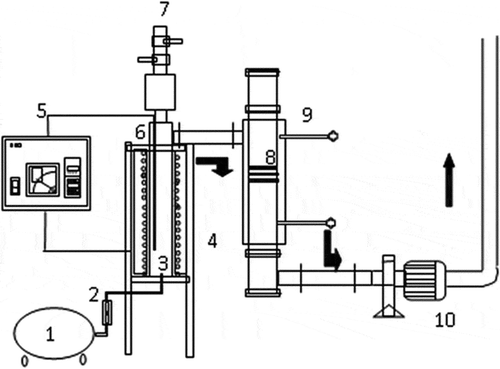
Table 1. Composition of feedstocks and operating conditions
Figure 2. Sampling train for PAHs: (1) sampling probe; (2) heated filter and heating hose; (3) thermometer; (4) cooling tube and XAD-4 adsorption tube; (5) 200 mL distilled water; (6) silica gel; (7) flow meter; (8) connect to vacuum pump.
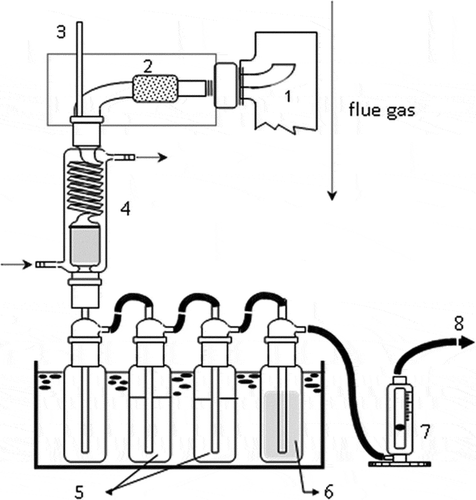
Table 2. Specific surface area and porosity of activated carbon fibers (ACFs) by N2 isotherms
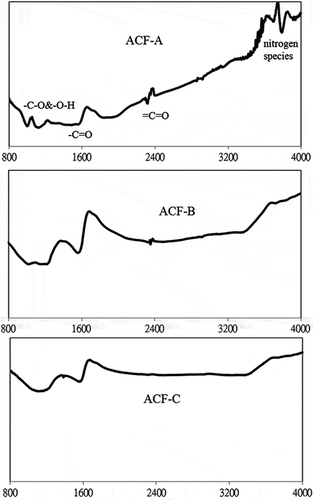
Table 3. Element content of the three activated carbon fibers (ACFs) by an elemental analyzer
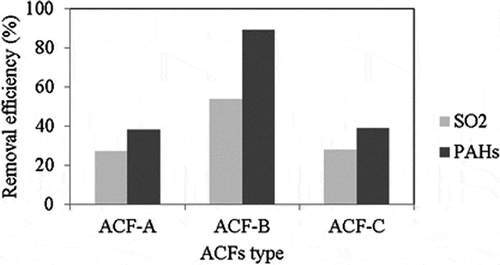
Figure 4. The knitted structure of a piece of activated carbon fibers (ACFs): (a) ACF-A; (b) ACF-B; (c) ACF-C (25×).
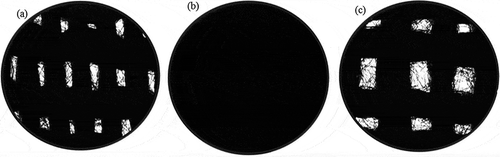
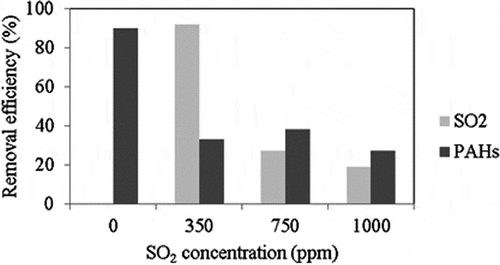
Figure 5. Effects of SO2 concentration on the removal efficiencies of SO2 and PAHs (reaction condition: reaction temperature = 200 °C, ACF type: ACF-A).