Figures & data
Figure 3. Effect of pH on Kd of Cs+/Sr2+ (50 mg/L Cs+/Sr2+ 20 mL, adsorbent: 100 mg activated porous calcium silicate, 2 hr).
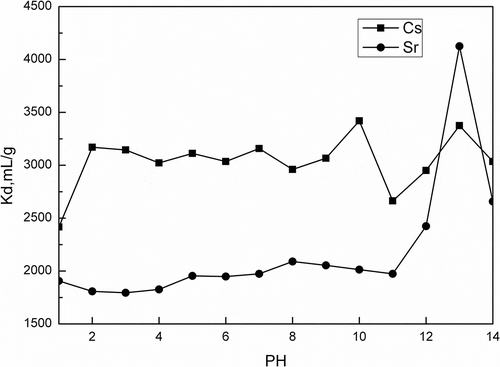
Figure 4. Effect of activated porous calcium silicate amount on Cs+/Sr2+ adsorption (50 mg/L Cs+/Sr2+ 20 mL, pH = 7.0, 2 hr).
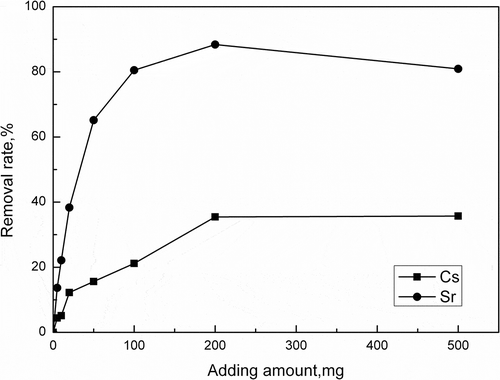
Figure 5. Effect of contact time on Cs+/Sr2+ adsorption (50 mg/L Cs+/Sr2+ 20 mL, pH = 7.0, 100 mg activated porous calcium silicate).
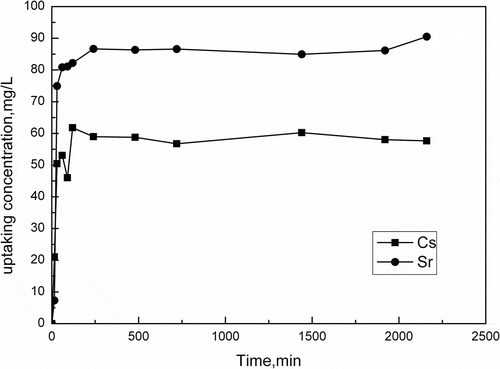
Figure 6. Effect of Cs+ /Sr2+ initial concentration on Cs+/Sr2+ adsorption (pH = 7.0, 100 mg activated porous calcium silicate, 2 hr).
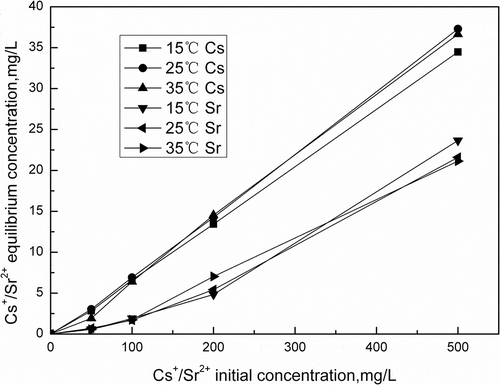
Table 1. Adsorption equilibrium data using Langmuir and Freundlich isotherm models
Table 2. Comparison of sorption capacity for Cs+ /Sr2+ by using various adsorbents
Table 3. Thermodynamic parameters for the adsorption Cs+/Sr2+ by activated porous calcium silicate
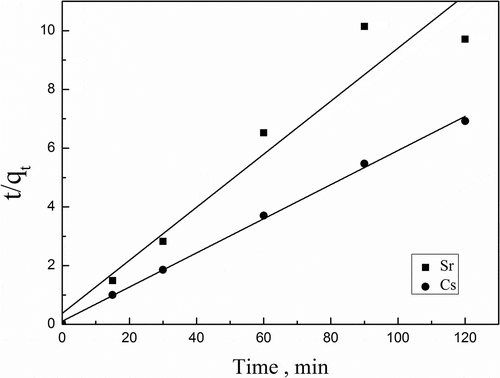
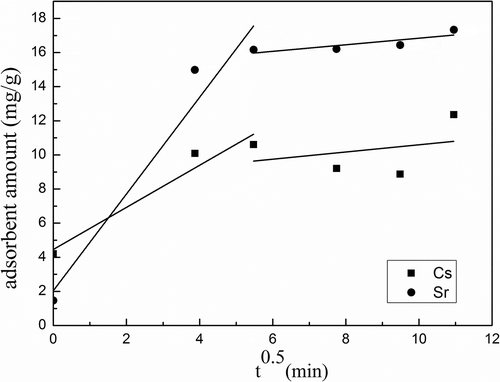
Table 4. The kinetic parameters of intraparticle diffusion model