Figures & data
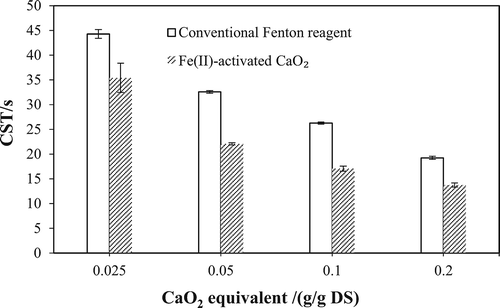
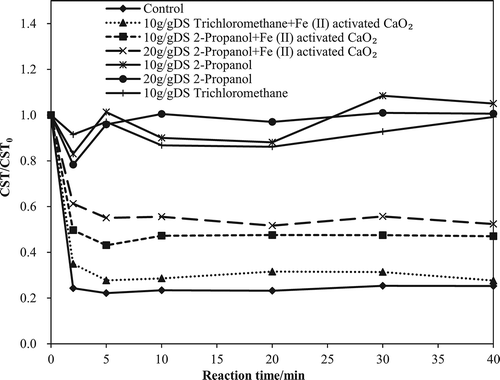
Figure 1. Performance of Fe(II)-activated CaO2 for enhanced dewatering of WAS. Left: CST; right: SRF. (a, b) Effect of CaO2 dosage on the dewaterability of WAS. (c, d) Effect of Fe(II) dosage on the dewaterability of WAS. (e, f) Effect of initial pH on the dewaterability of WAS.
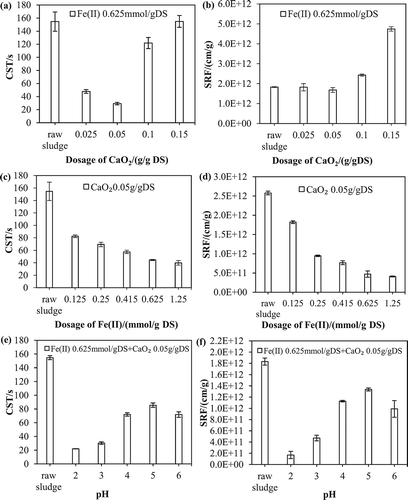
Figure 4. EPR spectra of radicals with DMPO spin trap in pretreated WAS: (a) conventional Fenton reagent; (b) Fe(II)-activated CaO2.
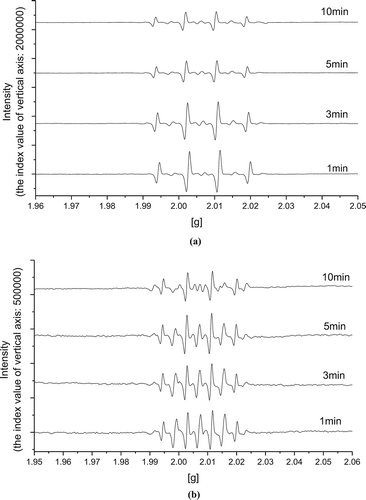
Figure 5. SEM spectra of WAS: (a) raw sludge; (b) pretreated sludge (working voltage: 10 kV; magnification: 25000×).
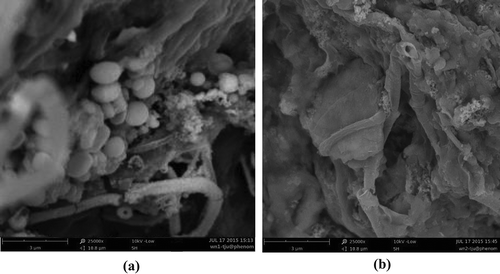
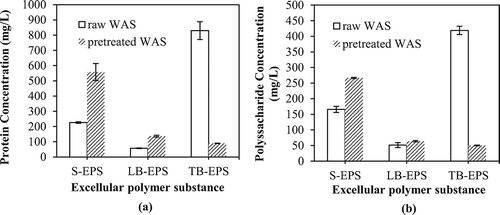
Figure 6. Molecular weight distribution of different EPS fractions: (a) S-EPS; (b) LB-EPS; (c) TB-EPS. (The conditioning concentrations were 0.05 g/g DS CaO2 and 0.625 mmol/g DS Fe(II) at an initial pH of 2.)
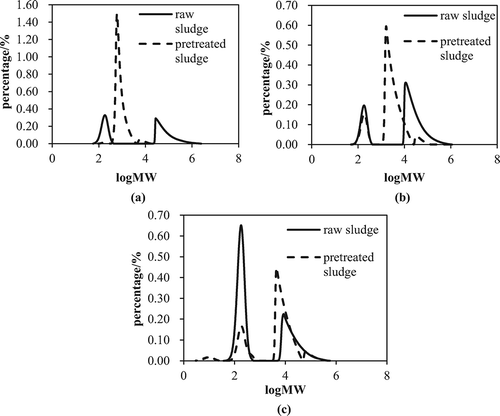
Figure 8. EEM spectra of different EPS fractions: (a) S-EPS of raw WAS; (b) S-EPS of pretreated WAS; (c) LB-EPS of raw WAS; (d) LB-EPS of pretreated WAS; (e) TB-EPS of raw WAS; (f) TB-EPS of pretreated WAS.
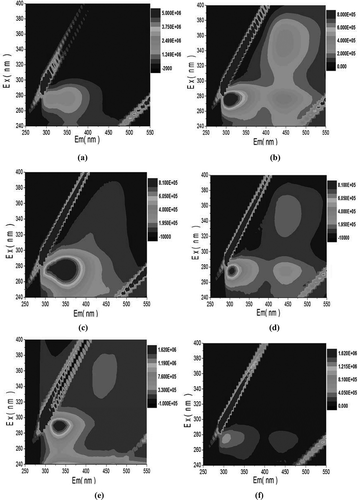
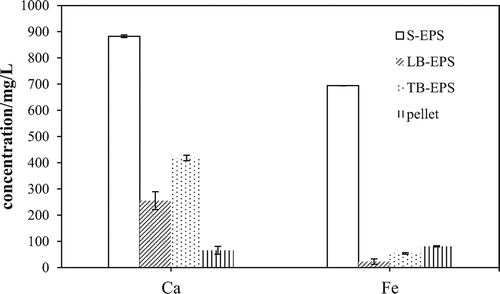
Table 1. Zeta potentials (mV) of different EPS fractions.