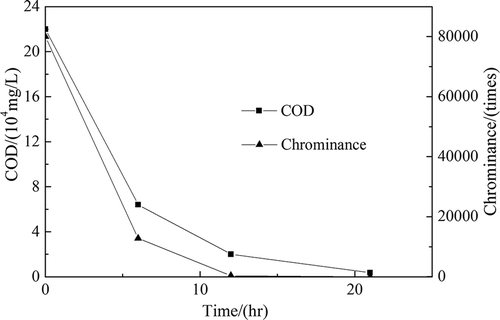
Figures & data
Table 1. Characteristics and components of waste sulfuric acid.
Table 2. Physical and chemical characteristics of activated carbon.
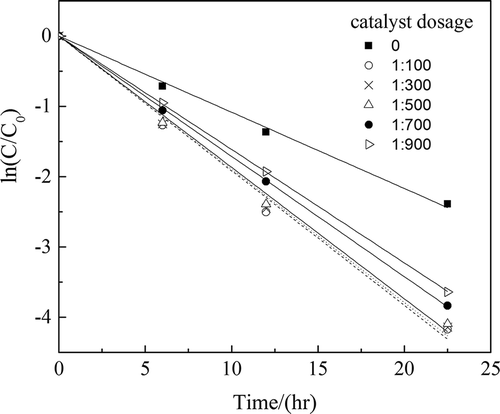
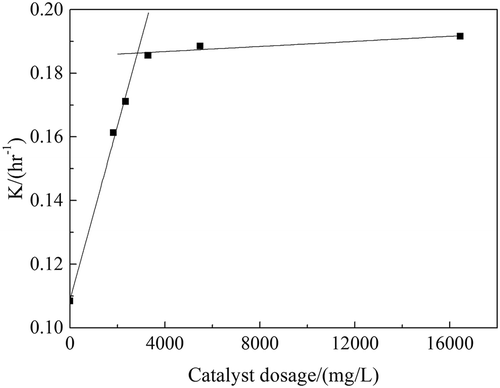
Table 3. K values at different dosage of catalyst.
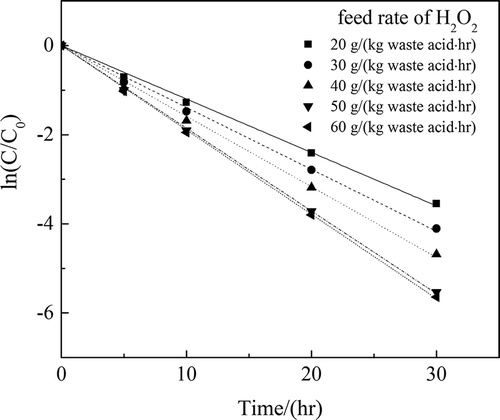
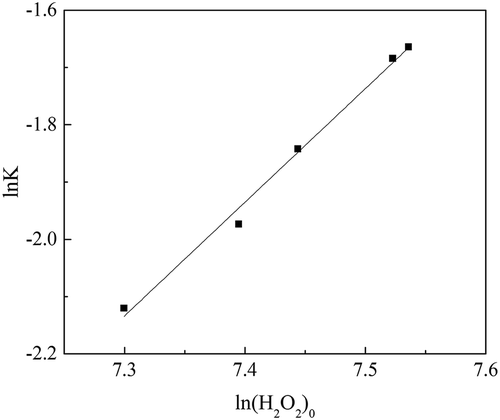
Table 4. K values at different rates of addition of H2O2.
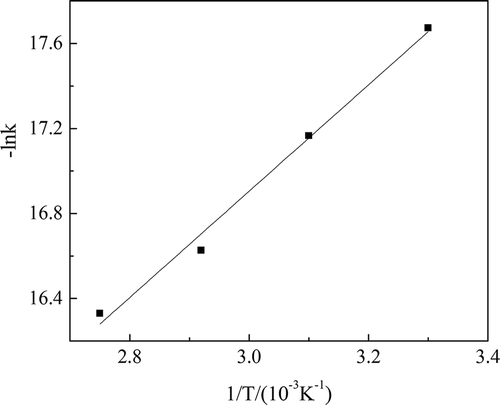
Table 5. Ln k values at different reaction temperatures.