Figures & data
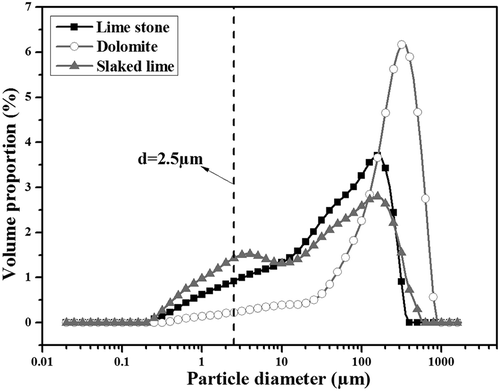
Table 1. Chemical compositions and proportion of raw materials in mixture (mass %).
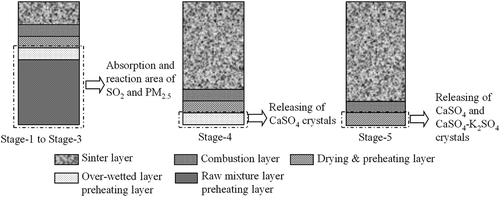
Figure 3. Typical changes of sinter layers during sintering process. (a) Gradual forming process of combustion layer and drying & preheating layer; (b) gradual forming process of overwetted layer; (c) formed overwetted layer; (d) gradual disappearing process of overwetted layer; (e) gradual disappearing process of drying & preheating layer; (f) gradual disappearing process of combustion layer.
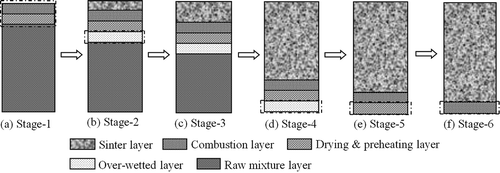
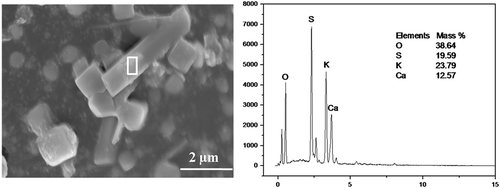
Figure 5. SEM-EDS of perfect crystallized and nonperfect crystallized CaSO4 crystals. (a) Morphology of perfect crystallized CaSO4 crystals; (b) EDX spectrum of perfect crystallized CaSO4 crystals; (c) morphology of nonperfect crystallized CaSO4 crystals; (d) EDX spectrum of nonperfect crystallized CaSO4 crystals.
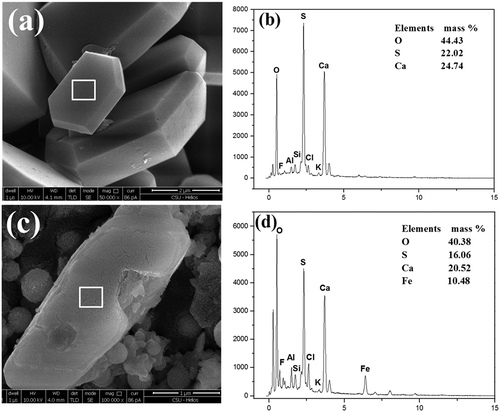
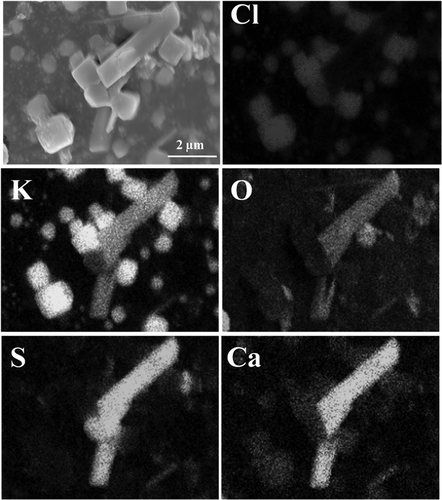
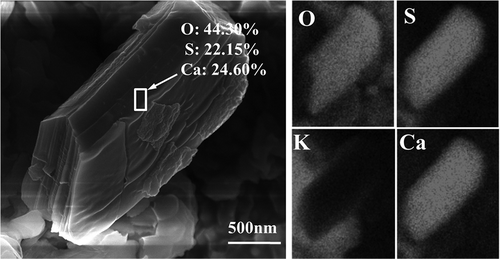
Figure 6. SEM-EDS of CaSO4-assembled particles. (a) EDX spectrum of selected area 1; (b) EDX spectrum of selected area 2.
Table 2. Removal ratio of K with increasing the ratio of coke breeze.