Figures & data
Table 1. The 50-species list for LU 3.0.1 combustion mechanism.
Table 2. C4 species and soot precursors.
Table 3. Comparison of prediction errors of reduced mechanisms for mole fraction of major species at residence time of 1 sec for C3H6 fuel (Wang et al., Citation2007).
Table 4. Comparison of prediction errors of reduced mechanisms for mole fraction of trace species at residence time of 1 sec for C3H6 fuel.
Table 5. Laminar flame speed (cm/sec)—Comparison of simulation and experimental results for propylene (Davis and Law, Citation1998).
Table 6. Laminar flame speed—Comparison of simulation and experimental results for methane (Law et al., Citation2006).
Table 7. Laminar flame speed—Comparison of simulation and experimental results for ethylene (Jomaas et al., Citation2005).
Figure 2. Laminar flame speed for different equivalence ratios for propylene (Davis and Law, Citation1998).
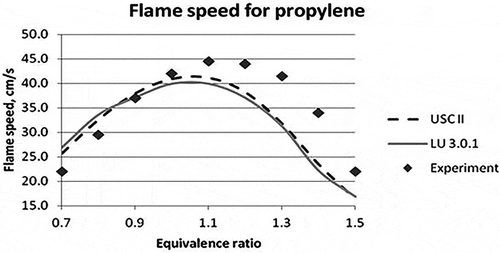
Figure 3. Laminar flame speed for different equivalence ratios for Methane (Vagelopoulos et al., Citation1994; Vagelopoulos and Egolfopoulos, Citation1998).
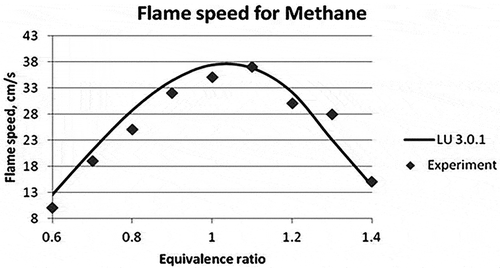
Figure 4. Laminar flame speed for different equivalence ratios for ethylene (Jomaas et al., Citation2005).
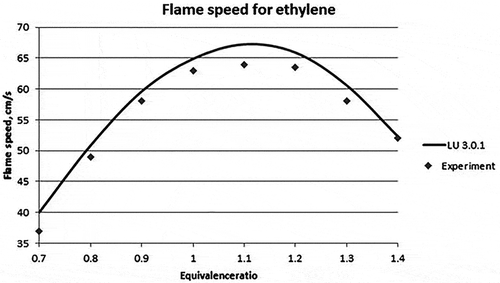
Table 8. Ignition delay—Comparison of simulation and experimental results.
Figure 5. Ignition delay time for propylene (Qin et al., Citation2001).
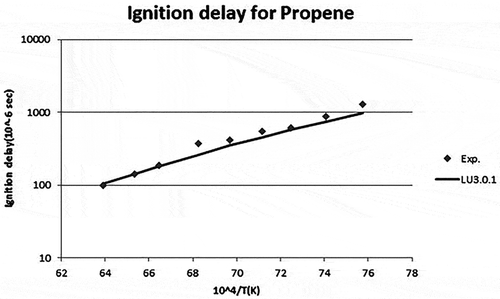
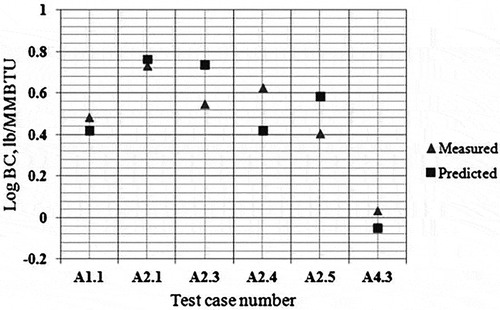
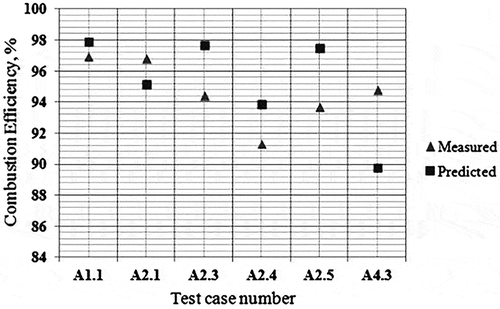
Table 9. Average percentage error of the reduced mechanisms compared with experimental data.
Figure 6. Adiabatic flame temperature of methane (Law et al., Citation2006).
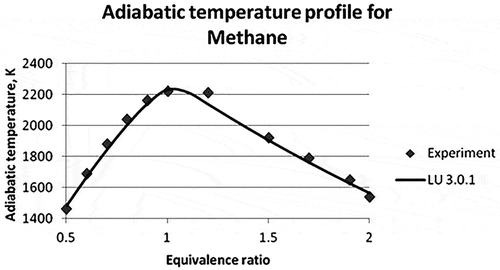
Figure 7. Adiabatic temperature profile of ethylene (Law et al., Citation2006).
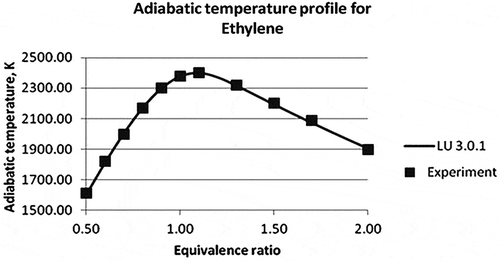
Table 10. Flamelet generation parameters.
Table 11. Grid refinement parameters for flamelet.
Table 12. Parameters used to generate the PDF table.
Table 13. Comparison of EDC and PDF models.
Table 14. Comparison of PDF and EDC model predictions.