Figures & data
Figure 1. Schematic of the TOA-QMS system for PM2.5 organic carbon and inorganic species measurement.
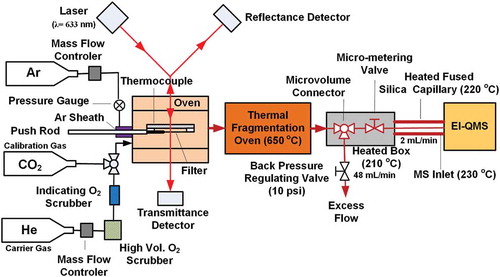
Figure 2. (a) Thermogram from burning of a birch log collected on a quartz-fiber filter comparing the IMPROVE_A temperature protocol with the simplified protocol used with the TOA-QMS; (b) TOA-QMS spectrum obtained for the 580°C portion from m/z 10 to 250, which constitutes most of the evolved gases. Major features are noted at m/z 27, 39, 44, 91, and 115.
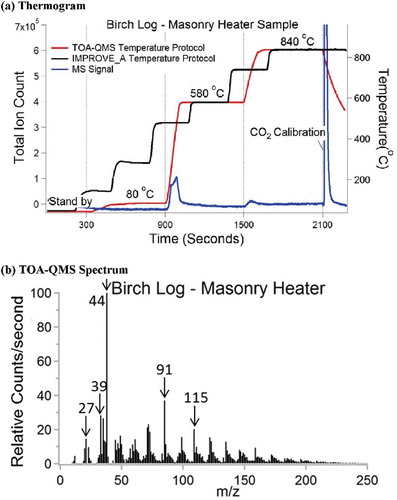
Figure 3. CO2 ionization efficiency (error bars of ±1 standard deviation derived from replicate analyses are smaller than the size of the dots).
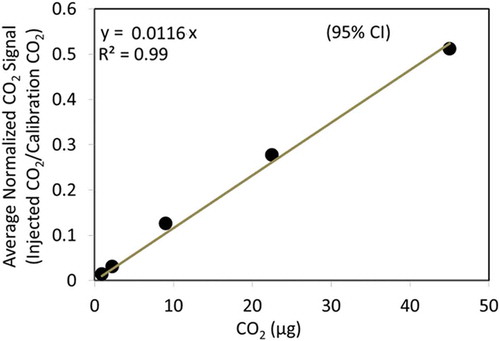
Figure 4. Calibration curves for (a) ammonium, (b) nitrate, (c) sulfate, and (d) organic carbon using oxalic acid (C2H2O4). Error bars indicates ±1 standard deviation derived from replicates. The y-axis shows the corresponding summed species m/z signals from the TOA-QMS normalized using the CO2 calibration peak m/z signal and divided by the filter punch area in cm2. The x-axis values were obtained from quantification by IC and TOA analysis of portions of the filter standards. Linear regression curves are forced through zero.
Figure 5. TOA-QMS and EI-AMS spectra for (a) NH4NO3, with the most abundant peaks at m/z 14, 15, 16, 17, 18, 30, and 46; (b) (NH4)2SO4, with the most abundant peaks observed at m/z 14, 15, 16, 17, 18, 32, 48, 64, 80, 81, and 98; (c) C2H2O4 with the most abundant peaks for TOA-QMS at m/z 29, 44, 45, and 46, and for EI-AMS at m/z 16, 17, 27, 28, 43, 44, and 45; and (d) CO2 with the most abundant peak at m/z 44.
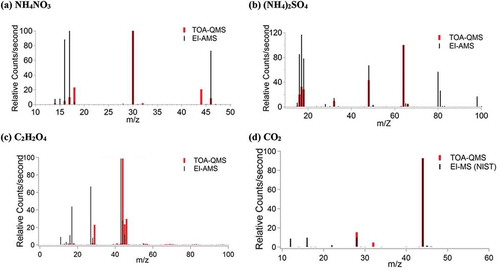
Table 1. Fragmentation patterns and interference corrections for ammonium, nitrate, and sulfate.
Figure 6. Spectrum processing flow diagram showing how interferences are removed from overlapping m/z signals.
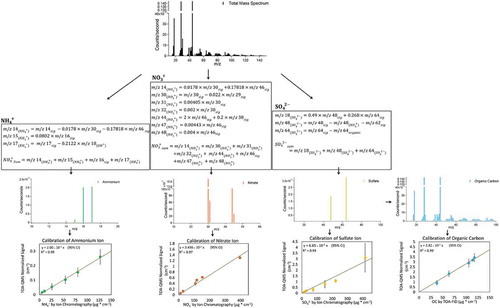
Figure 7. TOA-QMS concentrations vs. (a) NH4+ by IC, (b) NO3−. by IC, (c) SO42- by IC, and (d) OC by TOA for samples collected in Fresno, CA, and Baltimore, MD. Samples from the western United States, such as those from Fresno, CA, are rich in NO3−, while samples from the eastern United States, such as those from Baltimore, are rich in SO42-.
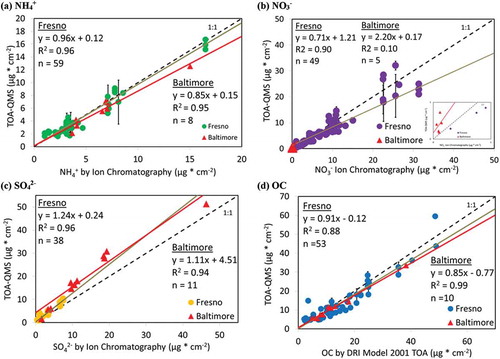
Figure 8. Average (colored bars) and standard deviations (black lines) of major chemical components in 58 Fresno, CA, samples measured by TOA-QMS, IC, and TOA. Elemental carbon (EC), soil, and salt concentrations determined from TOA, x-ray fluorescence (XRF), and atomic absorption spectrometry (AA), but not by TOA-QMS, are included for completeness. The NO3− offset is apparent, but the low SO42- averages are in reasonable agreement, as are the NH4+ and OC averages. The soil is calculated using the following formula: Soil = 2.2Al + 2.49Si + 1.63Ca +1.94Ti + 2.42Fe (Malm et al. Citation1994).
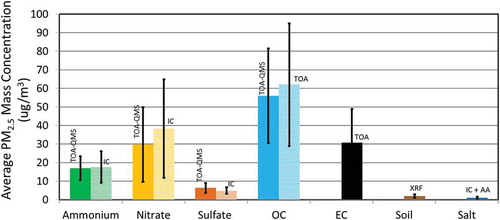
Figure 9. TOA-QMS mass concentration for (a) NO3− by IC adjusted with a factor of 1.36 plotted vs. IC mass concentration and (b) SO42- by IC adjusted with a factor of 0.80.
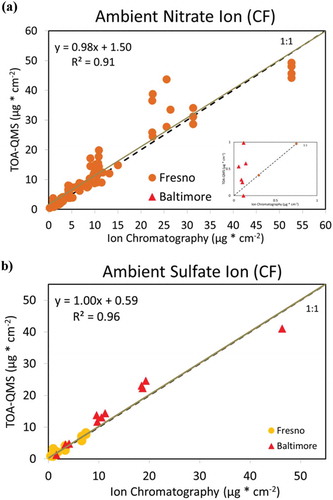
Figure 10. OC and ion contributions to PM2.5 determined by TOA-QMS at the Fresno Supersite from 12/15/200 to 02/03/2001. Five samples were collected for each day (i.e., 0000–0500, 0500–1000, 1000–1300, 1300–1600, and 1600–2400 local standard time [LST]). Highest concentrations of OC and NO3− were observed during the late afternoon to night time period (1600–2400 LST).
![Figure 10. OC and ion contributions to PM2.5 determined by TOA-QMS at the Fresno Supersite from 12/15/200 to 02/03/2001. Five samples were collected for each day (i.e., 0000–0500, 0500–1000, 1000–1300, 1300–1600, and 1600–2400 local standard time [LST]). Highest concentrations of OC and NO3− were observed during the late afternoon to night time period (1600–2400 LST).](/cms/asset/048d7e70-d7ee-4c58-8801-d234e5b4b19d/uawm_a_1394928_f0010_oc.jpg)
