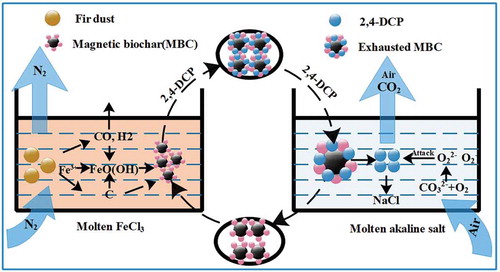
Figures & data
Table 1. Characteristic of FeCl3, 2,4-DCP and atrazine.
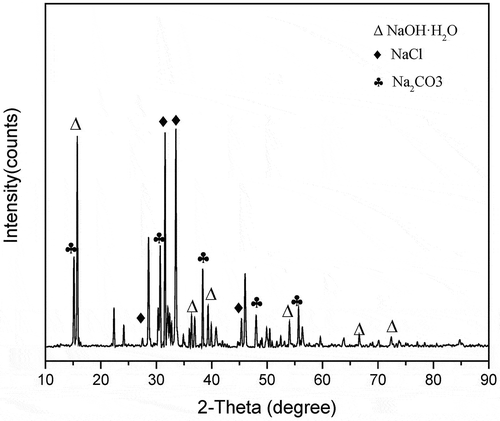
Table 2. Salt composition, melting points, and eutectic temperatures (Yao, Li, and Zhao Citation2011).
Figure 1. TG-DSC curves of the fir sawdust (a) and the fir sawdust mixed with FeCl3∙6H2O powder (b).
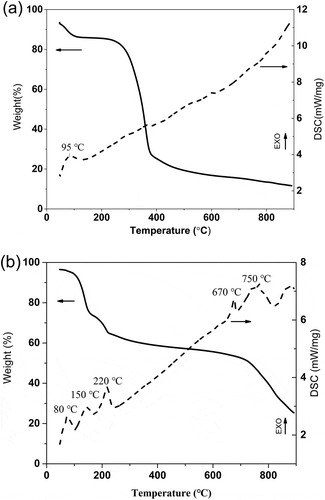
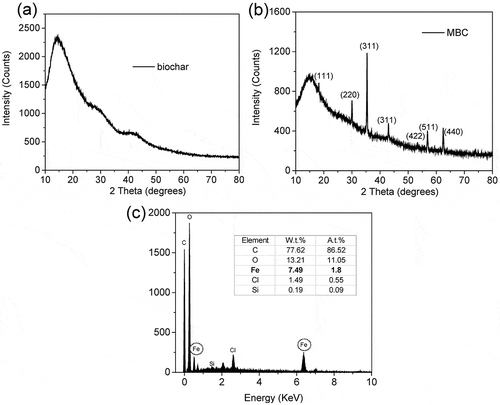
Table 3. Elemental analysis of MBC.
Figure 3. SEM images at different expansion ratios (a–d) and carbon, oxygen, and iron maps of MBC obtained by molten salt route (e–g). A color version is available online.
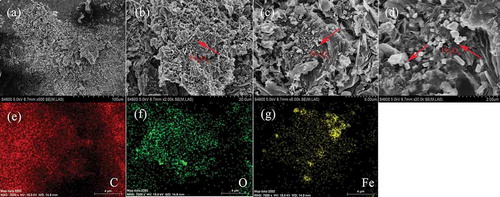
Figure 4. (a) Magnetic hysteresis loops of MBC. Digital image (inset) shows the magnetic separation by an ordinary magnet after 20 sec. (b) Raman spectrum of MBC. (c) N2 adsorption/desorption isotherms. (d) Pore size distribution.
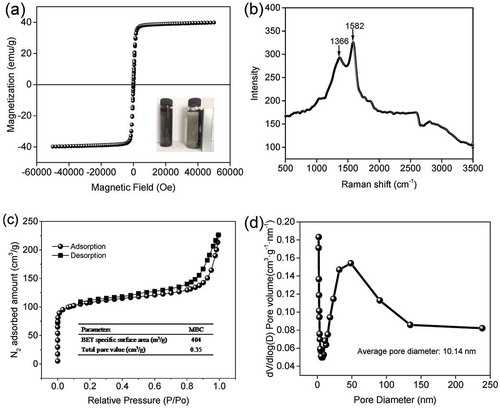
Figure 5. Adsorption kinetics for the 2,4-DCP (a) and atrazine (b) on MBC. Results of fitting experimental data to the first-order, the pseudo-second-order for the 2,4-DCP (c) and atrazine (d). Adsorption equilibrium isotherms for the 2,4-DCP (e) and atrazine (f). Initial concentration range, 20–200 mg L−1; temperature range, 10–30ºC; adsorbents dosage, 0.05 g; working volume, 50 mL; contact time, 24 hr.
Table 4. The content of Cl− and pesticides in the washing water and residues with molten NaOH–Na2CO3 method.
Table 5. Fixed carbon content, pore volume, and relative sample yield for the samples derived in molten salt systems at 400ºC.
Table 6. Absorption capacity of regenerated MBC in several cycles.