Figures & data
Figure 1. Experimental system schematic. 1. Nitrogen gas; 2. Ethylene; 3.Air; 4.Sulfur dioxide; 5.Selenium solution; 6.Micro peristaltic pump; 7.Rotor flow meter; 8.Quartz U-tube; 9. Thermostatic oil bath 10. IDF burner; 11.Air flow uniformplate; 12.Exhaust filter; 13.Quartz fiber filter; 14. Two-dimensional lift platform; 15.1 Absorption bottle (empty); 16.2 Absorption bottle (5% HNO3 + 10% H2O2); 17.3 Absorption bottle (silicone); 18.Ice bath; 19.Vacuum pump.
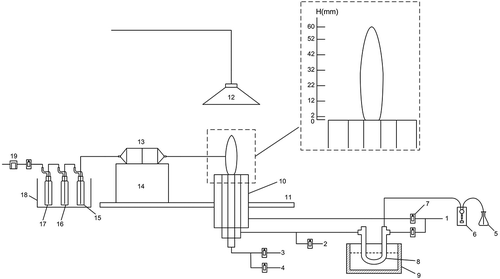
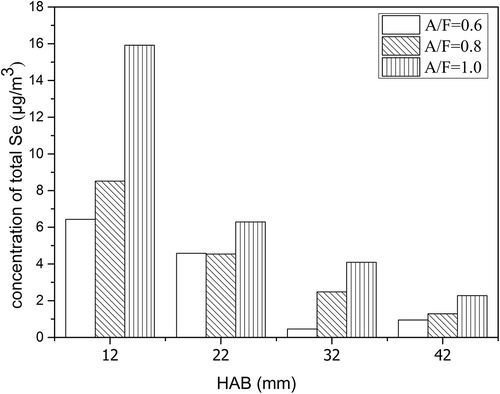
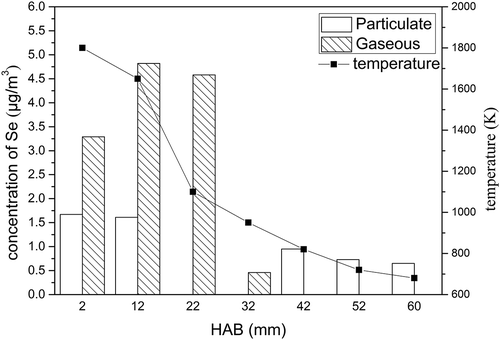
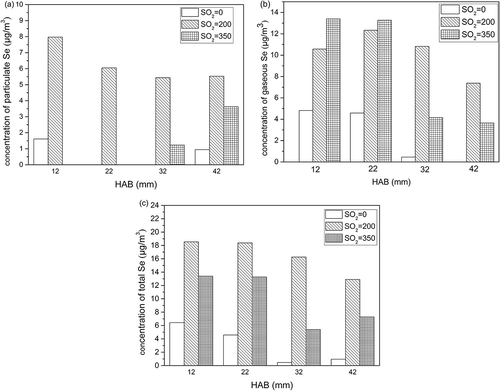
Table 1. The release of particulate selenium and gaseous selenium in different A/F ratios.