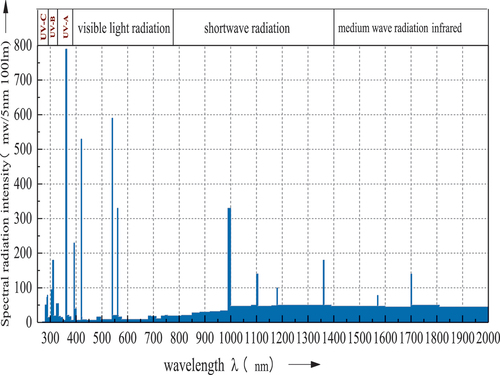
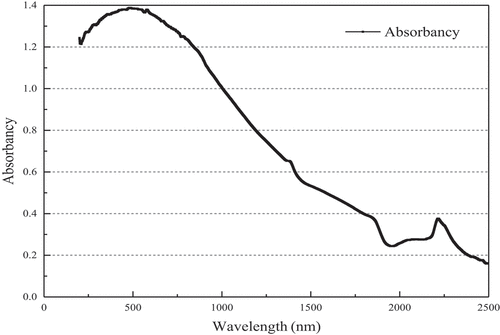
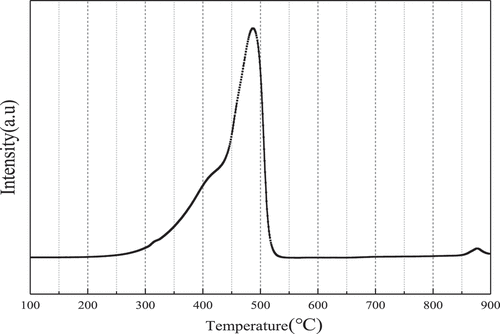
Figures & data
Figure 1. Experimental system diagram for formaldehyde purification performance testing 1. Formaldehyde generator; 2. Three-way valve; 3. Solar simulator; 4. Experimental cabin; 5. Stirring fan; 6. Catalyst carrier; 7. Catalyst; 8. Glass wall surface; 9. Formaldehyde detector; 10. Temperature sensor; 11. Vacuum pump; 12. Detection.
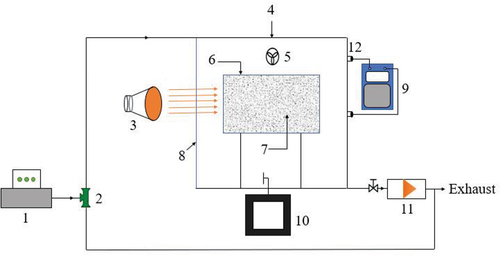
Figure 5. Scanning electron micrographs of the MnOx-CeO2 catalyst at 20,000 and 50,000
magnification.
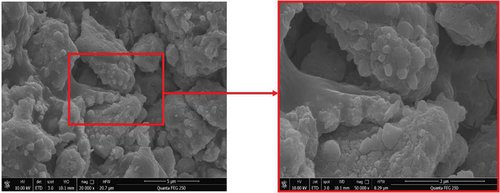
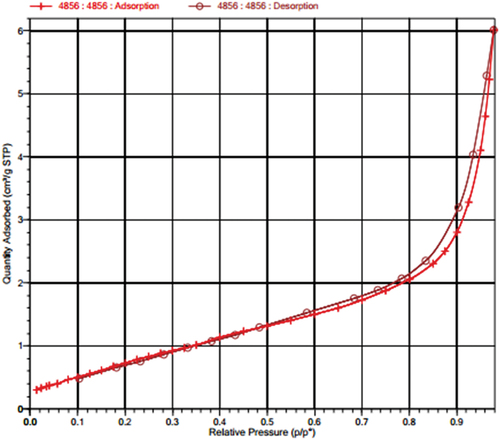
Table 1. Specific surface area, pore volume, and average pore diameter of the MnOx-CeO2 catalyst.
Table 2. Experimental condition table.
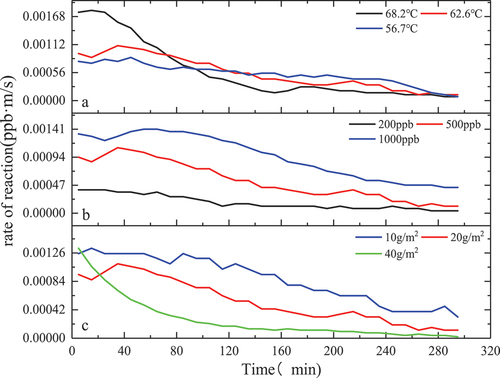
Figure 7. Reaction rate of MnOx-CeO2-catalyzed formaldehyde at different temperatures, concentrations and loadings.
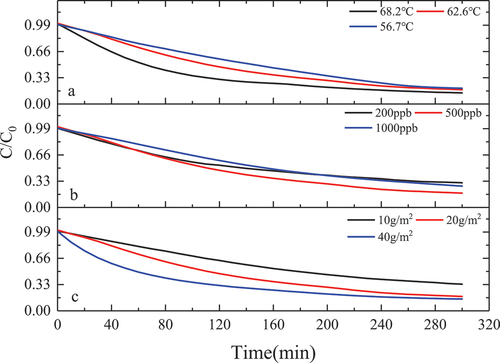
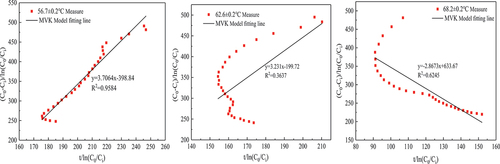
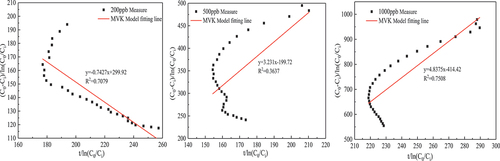
Table 3. MVK kinetic model parameters of thermal catalytic oxidation of formaldehyde.
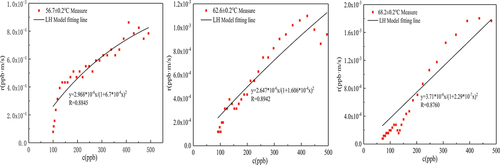
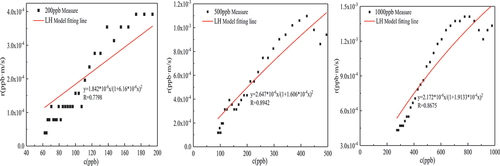
Table 4. LH kinetic model parameters of thermal catalytic oxidation of formaldehyde.
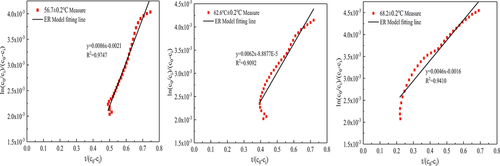
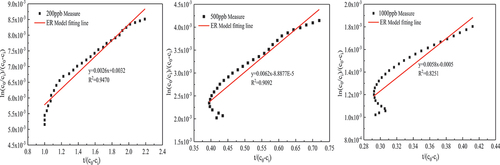
Table 5. ER kinetic model parameters of thermal catalytic oxidation of formaldehyde.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.