Figures & data
Table 1. Patient characteristics.
Table 2. Treatment efficacy in patients with anlotinib.
Figure 1. Prognostic factors associated with the progression-free survival after the administration of anlotinib. HR, hazard ratio; CI, confidence interval; P, p value.
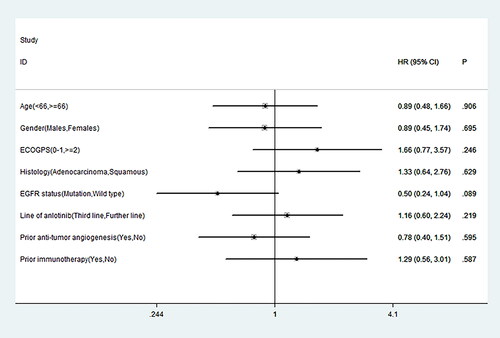
Figure 2. Prognostic factors associated with the overall survival after the administration of anlotinib. HR, hazard ratio; CI, confidence interval; P, p value.
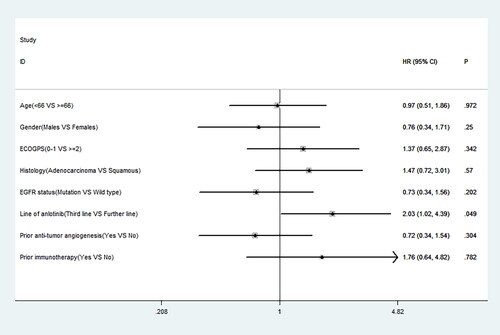
Figure 3. Progression-free survival and overall survival of patients who treated with anlotinib. (a) The median PFS, 4.0 months, 95% CI 2.92–5.08; (b) the median OS, 8.2 months, 95% CI 7.12–9.88.
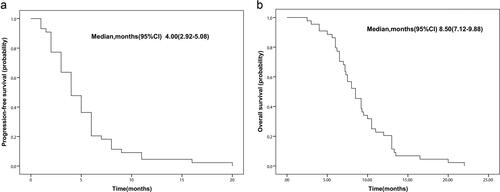
Table 3. Drug reactions in the anlotinib.
Data availability statement
All data are available by contacting correspondence authors: via: [email protected]
