Figures & data
Table 1. Central composite designed for optimization of sorafenib tosylate nanosuspension prepared by nanoprecipitation-sonication technique.
Table 2. Characterization of central composite designed sorafenib tosylate-loaded nanosuspension prepared by a nanoprecipitation followed by sonication technique.
Figure 1. FTIR spectrum of pure sorafenib tosylate (a), soyalecithin (B), Vit E TPGS (C), physical mixture (D), and optimized nanosuspension (formulation NSS6) (E).
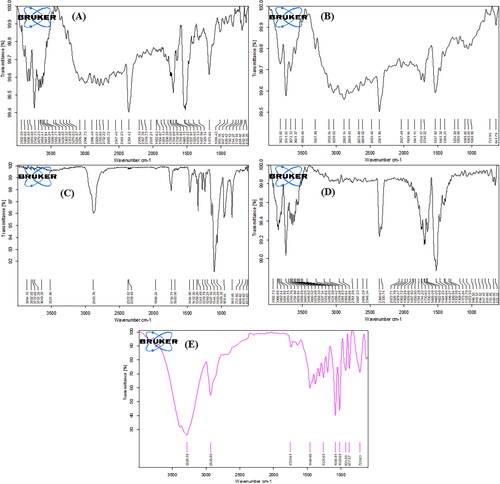
Figure 2. DSC thermogram of pure sorafenib tosylate (a), soyalecithin (B), TPGS (C), physical mixture (D), and optimized nanosuspension (formulation NSS6) (E).
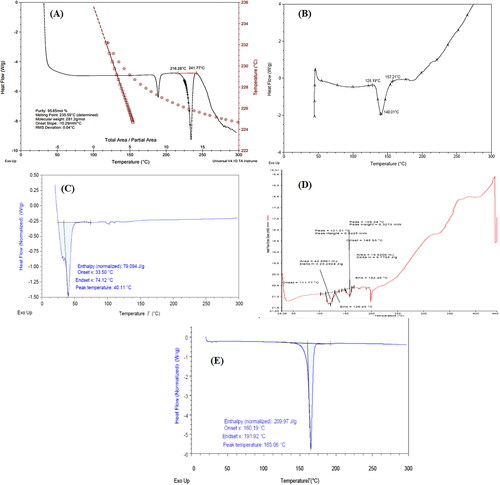
Figure 3. X-ray diffractogram of pure sorafenib tosylate (A) and optimized nanosuspension (formulation NSS6) (B).
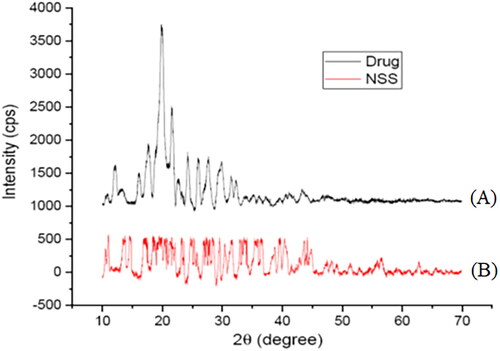
Figure 4. Scanning electron microscopic image of optimized lyophilized nanosuspension (formulation NSS6).

Figure 5. Particle size of formulation NSS6 (a), pareto chart represents particle size coefficients (b1, b2, b3 are main terms; b4, b5, b6 are interaction terms and b7, b8, b9 are square terms) (B), 3D response surface (C) and contour plot (D) showing impact of various process variables on particle size.
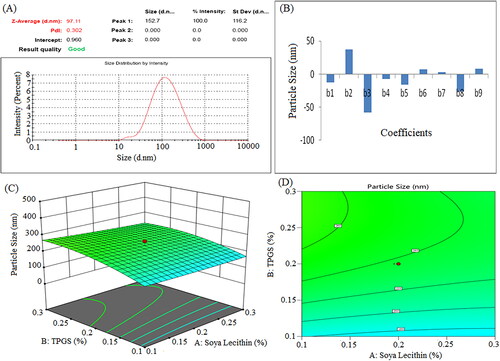
Figure 6. Zeta potential of formulation NSS6 (a), pareto chart represent coefficients of zeta potential (b1, b2, b3 are main terms; b4, b5, b6 are interaction terms and b7, b8, b9 are square terms) (B), 3D response surface plot (C) and contour plot (D) and showing impact of various process variables on zeta potential.
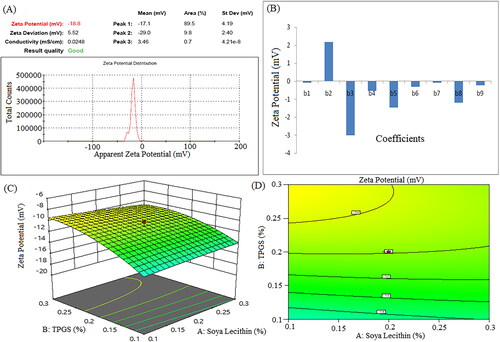
Figure 7. Pareto chart represent coefficients of drug content (b1, b2, b3 are main terms; b4, b5, b6 are interaction terms and b7, b8, b9 are square terms) (a), 3D response surface plot (B) and contour plot (C) showing impact of various process variables on drug content.
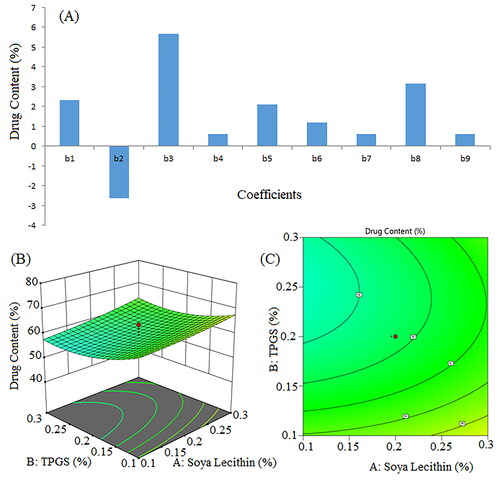
Figure 8. Pareto chart for coefficients of entrapment efficiency (b1, b2, b3 are main terms; b4, b5, b6 are interaction terms and b7, b8, b9 are square terms) (a), 3D response surface plot (B) and contour plot (C) illustrating the impact of various process variables on entrapment efficiency.
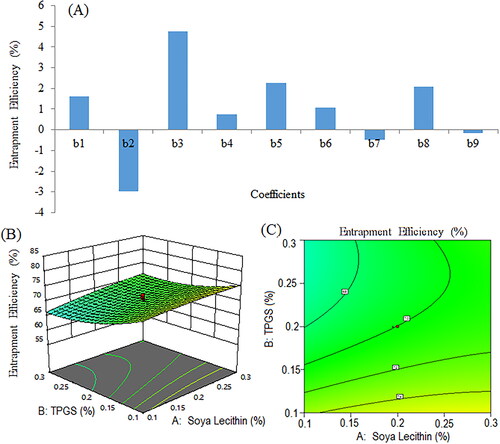
Figure 9. Pareto chart represents coefficients showing coefficients of percent cumulative drug release in 0.1 N HCl (pH 1.2) (b1, b2, b3 are main terms; b4, b5, b6 are interaction terms and b7, b8, b9 are square terms) (a), 3D response surface (B) and contour plot (C) and showing impact of various process variables on percent cumulative drug release.
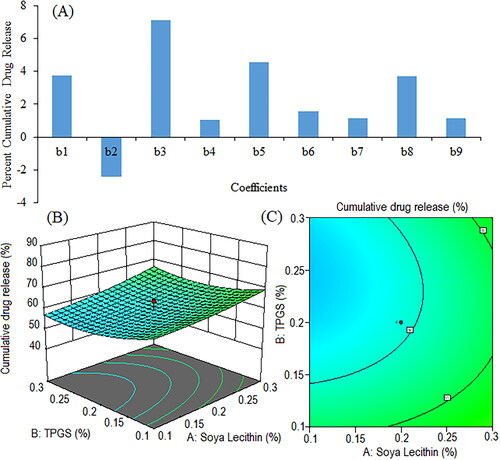
Figure 10. Pareto chart showing coefficients of percent cumulative drug release in phosphate buffer (pH 6.8) (b1, b2, b3 are main terms; b4, b5, b6 are interaction terms and b7, b8, b9 are square terms) (a), 3D response surface plot (C) and contour plot (C) illustrating the impact of various process variables on percent cumulative drug release.
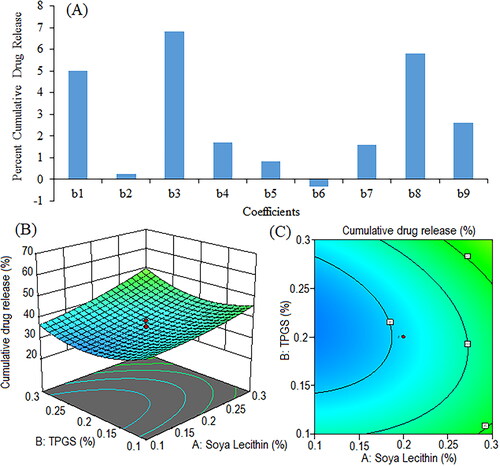
Table 3. Results Of two-way ANOVA of regression on the particle size, zeta potential, drug content, entrapment efficiency, and percent cumulative drug release in pH 1.2 and pH 6.8 dissolution media.
Figure 11. Cumulative percentage release of sorafenib tosylate in 0.1 N HCl (pH 1.2) from the nanosuspension prepared using nanoprecipitation-sonication technique.
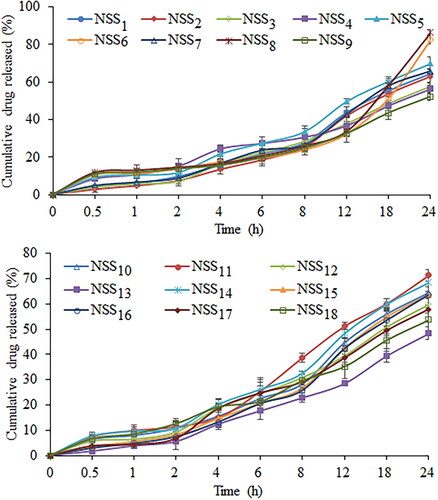
Figure 12. Cumulative percentage release of sorafenib tosylate in phosphate buffer (pH 6.8) from the nanosuspension prepared using nanoprecipitation-sonication technique.
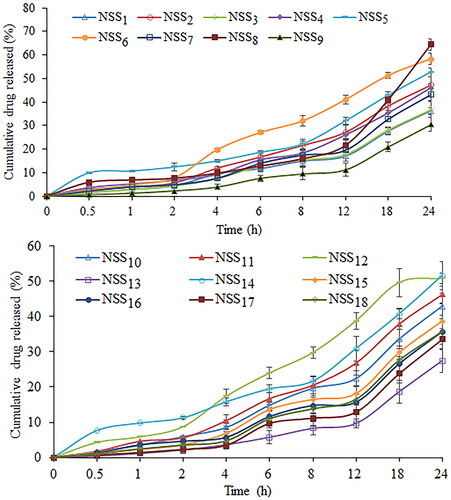
Table 4. Correlation coefficient value from different drug release kinetics.
Figure 13. Comparison of in vitro release of optimized batch and marketed formulation (Soranib, Cipla Ltd., Mumbai, India) in 0.1 N HCl and phosphate buffer.
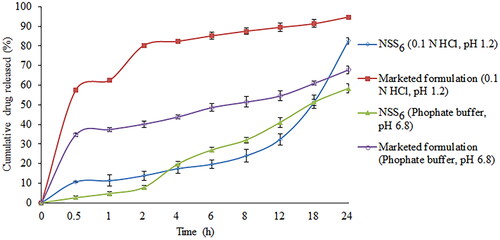
Table 5. Results of stability study of nanosuspension.