Figures & data
Figure 1. Scheme showing a starfish oocyte with part of the follicular cells which surround it. A ligand(the gonadostimuline, GS), in the reproductive season stimulates the secretion of the hormone 1‐methyladenine within the follicular cells. A peptide hormone, produced by the radial nerves, elicits 1‐methyladenine excretion from the follicular cells allowing it to bind the oocyte surface where it stimulates a G protein whose βγ subunits cause on one side cAMP decrease and on the other MPF activation with consequent germinal vesicle breakdown. With this breakdown there is further progress toward maturation until metaphase occurs, mediated by MOS and MAPK, but with no activation of the Na+/K+ antiporter, which is activated after oocyte shedding into seawater.
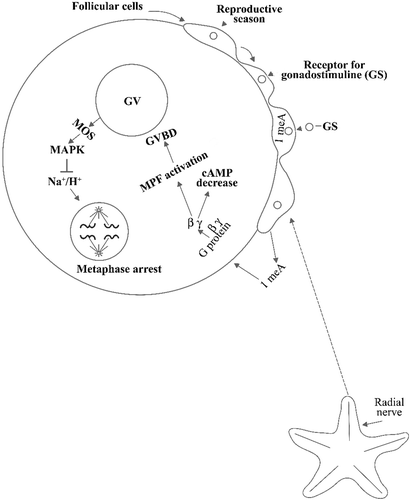
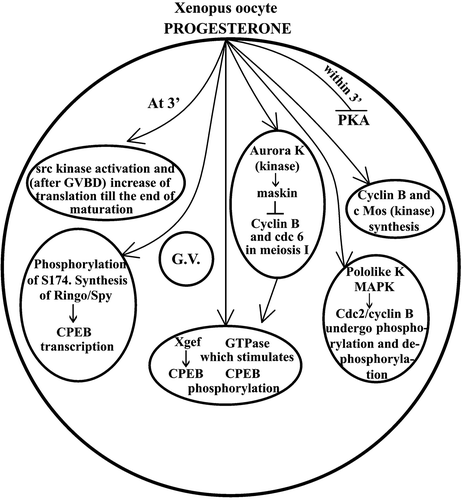
Figure 2. (a) Scheme showing the pituitary gonadotropin acting on the follicular cells surrounding the Xenopus oocyte and stimulating progesterone production. Progesterone acts in three ways: one, liberating Cdc25 from the 14‐3‐3 protein and moving it to oocyte pronucleus; second, binding its receptor and therefore inhibiting a G protein from breaking into its subunits; and third, by stimulating binding of the protein CPEB to its site (CPR) in the Mos mRNA. As a consequence of this, Mos mRNA becomes polyadenylated at its 3'end and capped at its 5'end, and therefore translates into its Pp39‐mos protein. This stimulates a MapKK, which in turn activates a MapK and with the aid of Hsp 70 and 90 activates the phosphatase Cdc‐25, which finally activates MPF. Lastly, MPF induces germinal vesicle breakdown. This moves the oocyte to what is described in Figure . (b) Histone phosphorylation which precedes germinal vesicle breakdown makes the oocyte proceed until the II metaphase at which point it becomes arrested again because of the sequential effects of P42, P‐90 RsK, CSF (cytostatic factor), and Emi‐1, until fertilization.
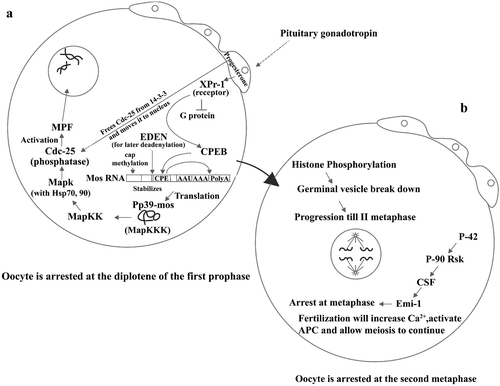
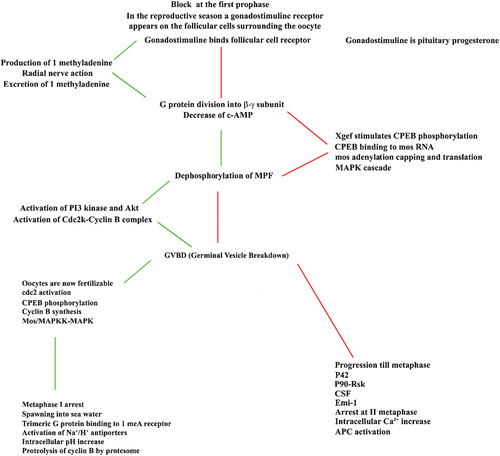
Figure 3. Comparison between the events leading to the maturation of oocytes of starfish, indicated on the left column, and of amphibians indicated in the right column: in the centre column the events common to the system are reported. The temporal sequence of events is from top to bottom and oblique segments indicate which events precede the other ones.