Figures & data
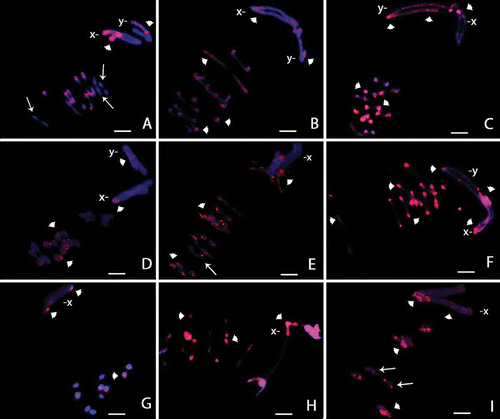
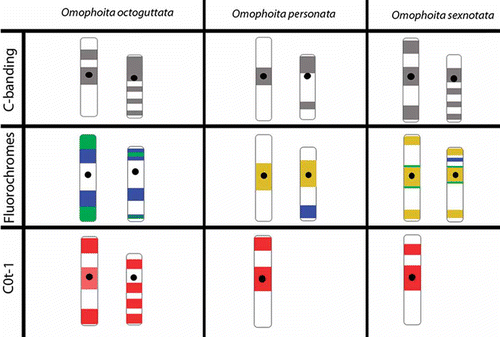
Figure 1. Meiotic cells from Omophoita octoguttata submitted to C-banding (A) and staining with the fluorochromes CMA3/DA/DAPI (B–H). A, metaphase I, arrow heads = centromeric positive staining, insert = sex chromosomes of another metaphase I and arrows show heterochromatin. B, DAPI positive = arrows. C, CMA3 positive = arrow heads. D, overlay of images B and C of the autosomes; arrows and arrow heads = positive staining. E, idiograms of the autosomes observed in D; arrow = AT-rich short arms and arrow head = GC-rich short arms. F, DAPI positive = arrows. G, CMA3 positive = arrow heads. H, overlay of images F and G of the anaphase II; arrows = AT-positive regions; arrow heads = GC-positive regions. Scale bar: 10 µm.
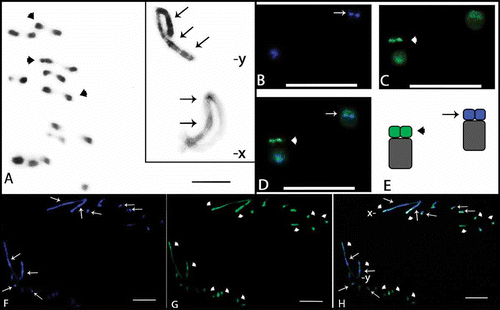
Figure 2. Meiotic cells from Omophoita personata submitted to C-banding (A) and staining with the fluorochromes CMA3/DA/DAPI (B–D). A, metaphase I; arrows = centromeric heterochromatin. B, DAPI positive = arrows. C, CMA3 positive = arrow heads. D, overlay of images B and C of the metaphase I, arrows = AT-positive regions and arrow heads = GC-positive regions. Insert = sex chromosomes (X and Y) of another cell. Scale bar: 10 µm.
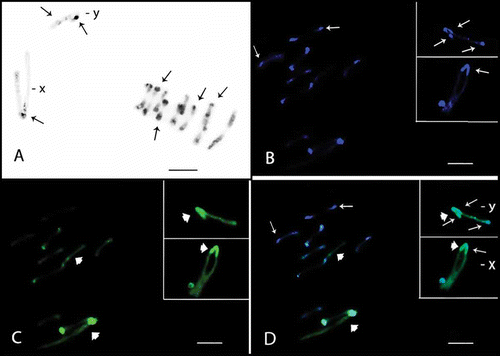
Figure 3. Meiotic cells from Omophoita sexnotata submitted to C-banding (A) and staining with the fluorochromes CMA3/DA/DAPI (B–D). A, metaphase I arrows = centromeric heterochromatin, insert = detailed view of the other Y chromosome. B, DAPI positive = small arrows. C, large arrows = CMA3 positive; and arrow heads = GC-positive short arms D, overlay of images B and C of the metaphase II, small arrows = AT-positive regions and large arrows = GC-positive regions. Insert = X and Y chromosomes; small arrows = AT-positive regions, large arrows = GC-positive regions and arrow heads = GC-positive proximal bands. Scale bar: 10 µm.
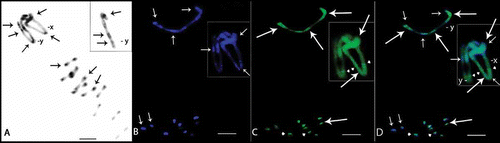
Figure 4. Meiotic cells from Omophoita octoguttata (A, D, G), O. personata (B, E, H) and O. sexnotata (C, F, I) submitted to cross-hybridisation using the total C0t-1 product from O. octoguttata in A–C, from O. personata in D–F, and from O. sexnotata in G–I. arrows = punctiform marks, arrow heads = positive marks. Scale bar: 10 µm.