Figures & data
Figure 1. The development of the cerebellum. A and B show the cerebellar anlagen in sagittal (A) and coronal (B) sections at E10 (hematoxylin and eosin staining). Cerebellum was developed from upper and inner rhombic lips (arrow heads) which connected with choroid (stars), and the space surrounded by the rhombic lips and choroid was the 4th ventricle (VT). Photo C shows P10 cerebellum with Nissl staining. Some cells (→) are located in the superficial external granular layer (EGL), while some granule cells have migrated into the internal granular layer (IGL). With cell migration, the cell density in the EGL became sparse, and there are few cells still remaining in the superficiality of the EGL or molecular layer. The area between the EGL and IGL is presumed to be the Purkinje cell layer (arrow head). Photo D shows the cerebellum at P14 (Tublin and NeuN double immunofluorescent labeling). A row of Purkinje cells (green) was seen in the Purkinje cell layer (PCL), and their dendrites extended into the EGL. The IGL contained numerous granule cells (red) underneath the PCL. Photo E shows the cerebellar lamination and cell migration at P7 with Ki67 (red) and NeuN (green) immunofluorescent labeling. At this age, the three-layered structure of cerebellar lamination was formed, and EGL and IGL could be clearly recognized. The Ki67 positive cells (arrow head) in EGL were migrating toward IGL to form the mature granule cells (green). Photo F shows the P10 cerebellum with functional presynaptic buttons (synaptophysin and calbindin double immunolabeling), which were located in EGL and IGL (red). These presynaptic buttons in EGL can be connected with the dendrites of Purkinje cells (green). Scale bars: A and B: 200 µm; C and D: 80 µm; E: 60µm; F: 30 µm.
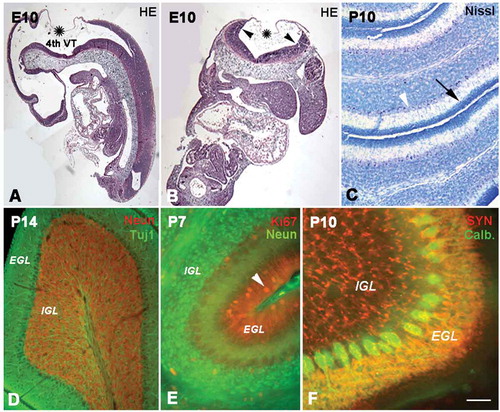
Figure 2. The development of radial glial cells in cerebellum. Photo A shows the radial glial cells (red) at E16 (Nestin immunolabeling), whose processes spanned from the ventricular zone (VZ) to the pia. However, their distribution patterns were quite different in different areas. In the superficial zone of the cerebellum, such as the external granular layer (EGL), the processes were arranged in an orderly radial pattern, but in the internal granular layer (IGL), their arrangement was in relative disorder. With age increase, the EGL became wide with the typical radial pattern for the processes of radial glial cells (B). Photo C shows the P7 cerebellum with Nestin and glial fibrillary acidic protein (GFAP) double immunolabeling. The structure of the cerebellum became more typical at P7. At this age, the radial glial cells expressed both Nestin (red) and GFAP (green). Photo D shows the radial glial cells (red) stained with S100 immunolabeling, and the Purkinje cells (green) were stained with calbindin immunochemistry. Photo E is the high magnification of photo D. At this age, the radial glial cells assumed the shape of typical Bergmann cells. Their cell bodies were located in the Purkinje cell layer (PCL), and the processes extended into the EGL. A high magnification of Bergmann cells (green) with cell bodies (arrow head) and processes (arrow) is also showed in photo F with S100 immunochemistry. Scale bars: A and B:, 100 µm; C: 30 µm; D: 60 µm; E: 25 µm; F: 15 µm.
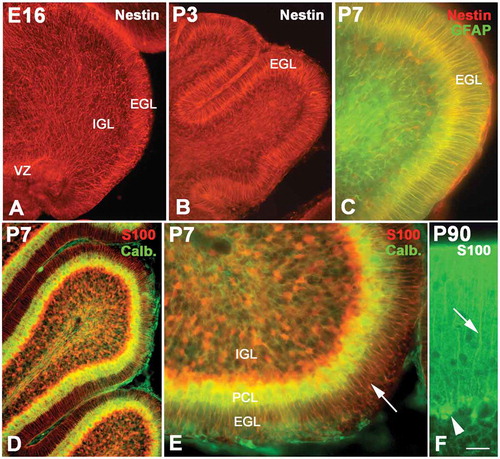
Figure 3. Neural migration in the cerebellum. Photos A–C, neural migration from the ventricular zone (VZ) to the Purkinje cell layer (PCL) (Nestin and Foxp2 double immunolabeling). At E15, numerous Foxp2 positive cells (green) migrated from VZ to PCL along the processes of radial glial cells (red) A, At E18, the migrating Foxp2-positive cells were concentrated near the PCL B, At P5 only one or two rows of Purkinje cells were located in the PCL C, Photos D–F, the migration of newborn neurons from the superficial external granular layer (EGL) to the internal granular layer (IGL). With double-cortin (DCX, red) and NeuN (green) double immunolabeling, photos D and E show that DCX-positive newborn neurons (red) in EGL are ready to migrate toward IGL and differentiate into granule cells (green). Photo F shows the cerebellar cortex at P10 (NeuN and Foxp2 double immunolabeling). There are few post-mitotic neurons in the superficial EGL (green, arrow), since almost all neurons have migrated into the IGL and differentiated into granule cells (green). The Purkinje cells (red and arrow head) are visualized between EGL and IGL. Photos G–H, neural proliferation and neural migration in the cerebellum (5-Bromo-deoxyuridine (BrdU) assay). The proliferative cells and migrating neurons (green) decreased with age increase. The proliferative cells in EGL are marked with arrows. At P14, only a few proliferative cells were located in the superficial zone of EGL (I). Scale bars: A and B: 100 µm; C: 50 µm; D and E: 100 µm; F: 50 µm; G–I: 100 µm.
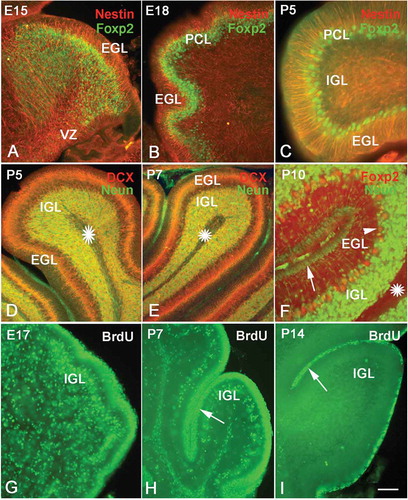
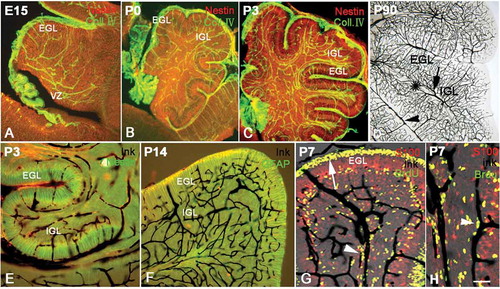
Figure 4. The development of vasculature and radial glial cells in the cerebellum. Photos A–C, the distribution of vessels and radial glial cells at various ages with Collagen IV (green) and Nestin (red) double immunolabeling. The vascular network (green) appeared in the cerebellum as early as E15. The vasculature in the presumable superficial external granular layer (EGL) was orientated radially and paralleled with the processes of radial glia (red) in same area. However, the vasculature in the internal granular layer (IGL) appeared disorderly; so did the processes of the radial glia in the same area. At P0 and P3, the vasculature became dense, and their distributions were still highly identical with the arrangement of processes of radial glia. Photo D shows the vascular network at P90 with ink perfusion (dark). The vasculature in white matter is marked by a star, and the relatively large vessels in the pia and white matter are marked by an arrowhead and arrow respectively, showing that the cortical capillary network converges into the vessels in the pia and white matter. Photos E–F, cerebellar vasculature and the development of radial glial cells by ink perfusion and Nestin or glial fibrillary acidic protein (GFAP) immunochemistry. The orientation of vessels (dark) was highly harmonized with the distribution of the processes of radial glia (green) as described above, again suggesting the close interaction between radial glial cells and blood vasculature in the cerebellum. Photo G, cerebellar vessels and neural migration by ink perfusion, S100 immunochemistry and 5-Bromo-deoxyuridine (BrdU) assay triple labeling. The proliferative BrdU-positive cells were mainly concentrated in the subventricular zone (SVZ) and EGL. Since BrdU injections were carried out 2 days before animal sacrifice, some BrdU-positive neural progenitors had differentiated into post-mitotic neurons and migrated elsewhere. In the photo, many BrdU-positive migrating cells were observed along the cerebellar vessels (arrowhead), suggesting that the cerebellar vasculature served as a migration scaffold similar to the function of radial glial cells. The arrow shows the proliferative area in EGL, which are the source of migrating neurons into IGL. Photo H, the neural migration under vascular guidance with high magnification using triple labeling (ink, S100 and BrdU). Many BrdU-positive cells were migrating along the vascular scaffold as described above (arrow head). Many BrdU-positive cells (green) expressed S100 (red) with a merged yellow color, suggesting that proliferative stem cells were transiting toward newborn post-mitotic neurons. Scale bars: A–C: 200 µm; D–F: 100 µm; G: 60 µm; H: 40 µm.