Figures & data
Figure 1. Notch1 and Purkinje cell differentiation. A, Neurulation and cerebellar development at embryonic day 12 (E12) (Sox2 (Sex-determining-region-Y-related high-mobility-group-box protein-2) and Nestin immunolabeling). Rhombic lips (RL) are the anlage of cerebellum. Lateral ventricle (LV), cerebral aqueduct (CA) and somites are marked. B–E, Neural progenitor cell and Purkinje cell differentiation. With Nestin (red) and Sox2 (green) immunolabeling, the radial glial cells and neural progenitor cells were found at age E9 (B). The radial glial cells appeared radially around the cavity (stars) of the neural tube, and the neural progenitors were located in the neural epithelium. The radial glia extended apical and basilar projections toward the pia and the lumen. At E15, rhombic lip thickened and became large (C), and newborn Purkinje cells started to migrate from the ventricular zone (arrow head) to the Purkinje cell layer (PCL). With Nestin & Foxp2 (forkhead box p2) double immunolabeling, at this age, numerous Foxp2 positive cells (green) have migrated from ventricle zone (VZ) (arrowhead) to PCL along the processes of radial glial cells (red; C). The external granular layer could be recognized (arrow) in the figure without Foxp2 positive cells inside. At postnatal day (P0), the migrating Foxp2 positive Purkinje cells were mainly concentrated in PCL with 3–4 rows (D). With Calbindin immunolabeling, only one or two rows of Purkinje cells have been located in PCL after P5 onward (E). The molecular layer (ML) and cortical sulcus (star) are marked in the photo. F–G, Notch pathway and neural progenitor cells in neural tube. Hes1 (green), a downstream protein in Notch pathway, could be expressed in neural progenitor cells (green) around the cavity (star) of the neural tube at E9 (F). Notch1 (green) could be also found in cell bodies of neural progenitors among which are the Nestin positive radial glia (red) at E12 (G). H–I, Notch1 expression and Purkinje cell differentiation after postnatal day. With Notch1 and NeuN double immunolabeling, Notch1 is expressed strongly in Purkinje cells (green) above granular layer (GL, red) and interneurons in molecular layer at P14 (H). In adult, using Calbindin (red) and Notch1 (green) immunolabeling, Notch1 is expressed in mainly cell bodies of Purkinje cells, but no expression in the dendrites (arrowhead, red; I). Scale bars: A = 400 μm; B, F = 35 μm; C = 100 μm; D, E, H = 50 μm; G = 25 μm; I = 20 μm.
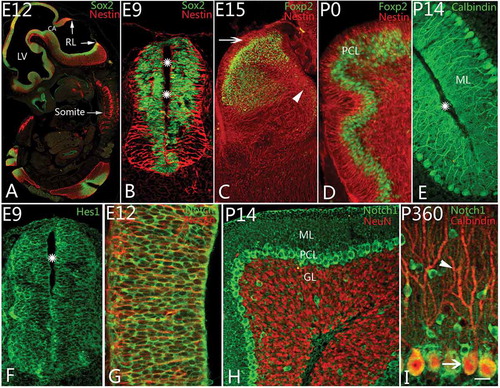
Figure 2. Shh expression and the differentiation of Bergmann cells. A, At postnatal day 3 (P3), Sonic Hedgehog (Shh) protein (green) could be found in Bergmann cells. In the photo, their cell bodies are arranged with one row (arrows) in the Purkinje cell layer. The molecular layer and cortical sulcus are marked with ML and stars, respectively, as well. B, At P14, with GFAP (glial fibrillary acidic protein) and Calbindin double immunolabeling, radial glia and Purkinje cells can be shown. The GFAP-positive radial glial cells are similar to the Shh-positive cells in their shape or location, suggesting that they belong to same kind of cells. The molecular layer and sulcus are marked with ML and stars as well. C, Shh can be expressed in Bergmann cells and the matrix of molecular layer (ML) at P14. The arrow points to the cell body of an Shh-positive cell. D, With Ki67 and Shh double immunolabeling, the neural progenitor cells (red) in ML were presumed to migrate into IGL along Bergmann cells (green). Scale bars: A, B= 50 μm; C = 20 μm; D = 25 μm.
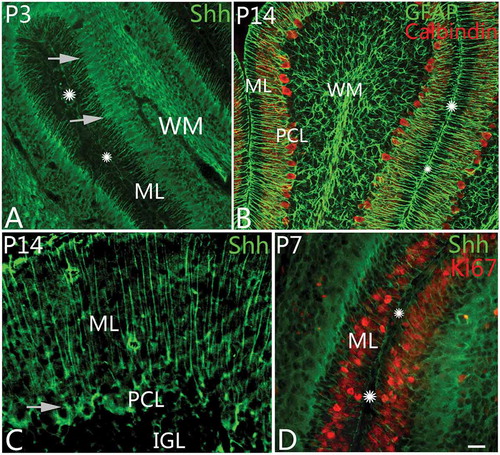
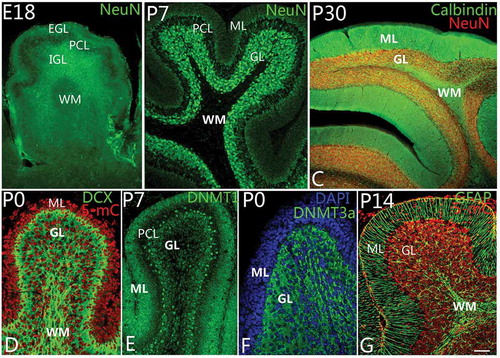
Figure 3. Differentiation of granule cells and DNA methylation. A–C, Granule cell differentiation and migration. With neuronal nuclei (NeuN) immunolabeling, the newborn neurons in the external granular layer (EGL) could be observed to migrate into the internal granular layer (IGL) as early as at embryonic day 18 (E18) (A). The white matter (WM) located in the deep IGL was marked. At P7, almost all neurons in the molecular layer (ML) had migrated into the granular layer (GL) completely (B). The WM is a cell-free zone (NeuN immunolabeling). At P30, with NeuN and Calbindin double immunolabeling, cerebellar lamination had been formed typically with ML, Purkinje cell layer, granular layer (GL) and WM (C). D–F, DNA methylation during cerebellar development. At P0, with 5-methylcytosine (5-mC) and Doublecortin (DCX, green) immunolabeling, 5-methylcytosine (red) can be expressed in the cell nuclei of both ML and GL (D). The expression of DNA methyltransferase 1 (DNMT1) showed the same pattern as 5-mC (E). It suggests that 5-mC and DNMT1 are mainly involved in cell proliferation in the lineage of granule cells. Interestingly, DNA methyltransferase 3a (DNMT3a) could be mainly expressed in the cytoplasm of granule cells in GL rather than in ML (F), suggesting DNA methylation has a close relationship with the development and differentiation of granule cells. The cells in ML and GL were counterstained with Dapi (blue). In G, Bergmann cells (green) are shown to arrange radially in the ML with Nestin immunolabeling. With cerebellar development, ML become a cell-free zone, due to the migration of neurons (red) into GL (G). Scale bars: A, C = 100 μm; B = 50 μm; D–G = 25 μm.