Figures & data
Table 1. USDA and its metal complexes.
Table 2. Cytotoxicity (CC50, μmol/L) of UDCA and its metal complexes against chicken hepatoma and rat sarcoma cell lines.
Table 3. Cytotoxicity (CC90, μmol/L) of UDCA and its metal complexes against chicken hepatoma and rat sarcoma cell lines.
Table 4. Cytotoxicity (CC50, μM) of UDCA and its metal complexes against human tumour and non-tumour cell lines.
Table 5. Cytotoxicity (CC90, μM) of UDCA and its metal complexes against human tumour and non-tumour cell lines.
Figure 1. Concentration–response curves of UDCA and its metal (Zn, Cu, Ni) complexes against LSCC-SF-Mc29 chicken hepatoma cells evaluated by an MTT test after 24 h (a), 48 h (b) and 72 h (c) treatment periods.
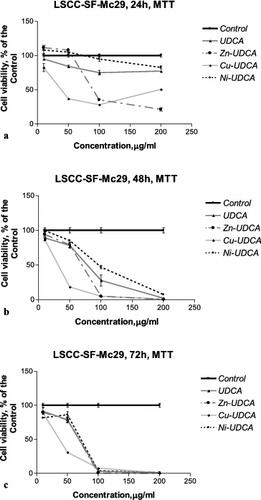
Figure 2. Concentration–response curves of UDCA and its metal (Zn, Cu, Ni) complexes against rat sarcoma LSR-SF-SR cells evaluated by an MTT test after 24 h (a), 48 h (b) and 72 h (c) treatment periods.
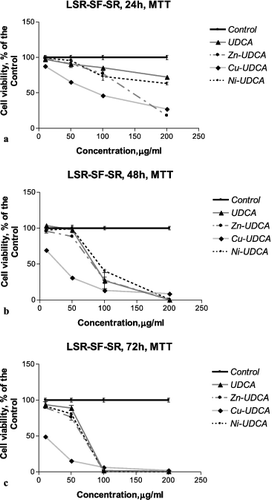
Figure 3. Concentration–response curves of UDCA and its metal (Zn, Cu, Ni) complexes against human A549 lung cancer and non-tumour Lep-3 cells evaluated by an MTT test after a 72 h treatment period.
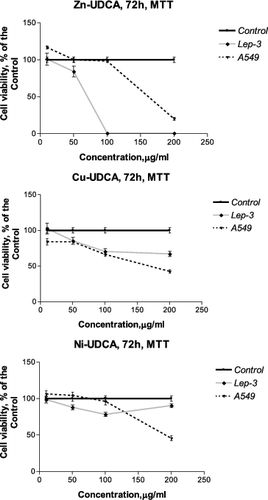
Figure 4. A complete monolayer of HeLa cells with a pale green nuclear fluorescence, bright yellow–green nucleoli as well as considerably more dull green fluorescence of the cytoplasm. (a) The cytoplasm includes focal perinuclear lysosomal accumulations with granular bright orange–red fluorescence. (b) HeLa cells 72 h following the treatment with 200 μg/mL UDCA. (c) 200 μg/mL Ni‒UDCA. (d) 200 μg/mL Cu‒UDCA. (e) 200 μg/mL Zn‒UDCA. Foamy vacuolation of the cytoplasm (b, c and d). Significant cell losses as well as intact and apoptotic dead cells in the (e) treatment. Acridine orange‑-propidium iodide staining.
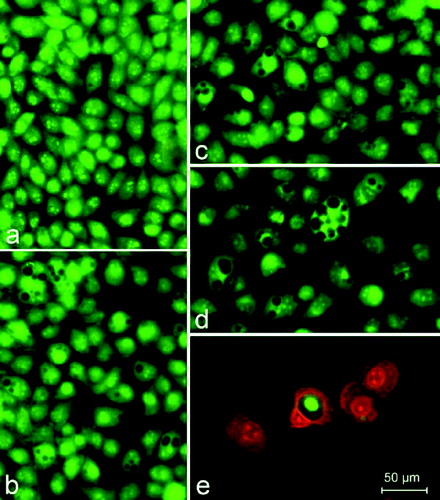
Table 6. Effect of UDCA and its metal complexes on colony-forming ability of tumour cells (CIC, μmol/L).
Table 7. Hierarchical orders of the compounds investigated according to their cytotoxic and/or antiproliferative activities.
