Figures & data
Table 1. Synthesized coding sequences and primers used for the construction of GB1–hEGF fusion plasmid.
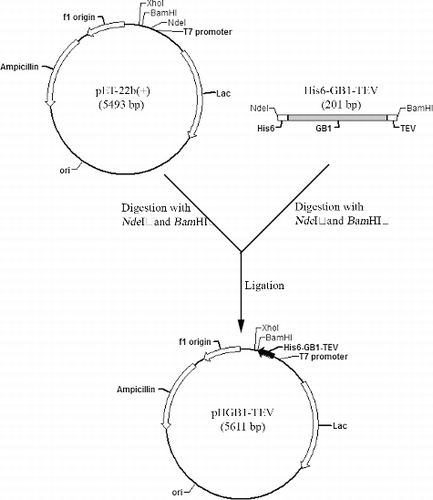
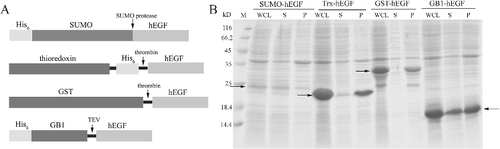
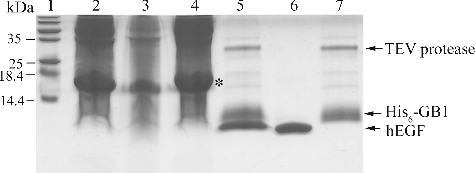
Figure 1. Engineered fusion sequence containing GB1, N-terminal hexahistidine (His6) tag and C-terminal TEV protease recognition site.