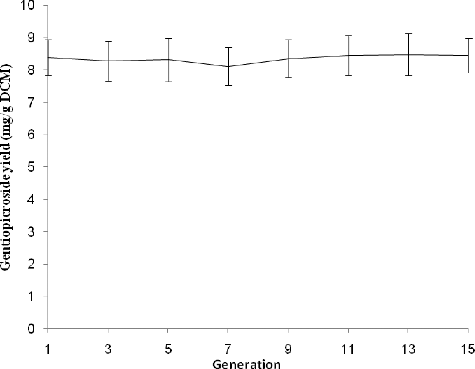
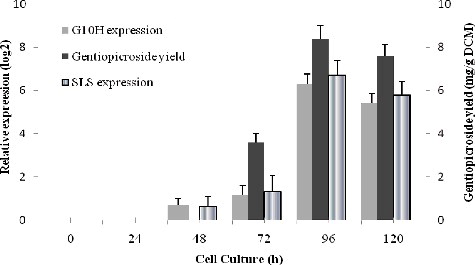
Figures & data
Figure 1. Representative PCR analysis of the G10H gene in yeast transformants. Lane M: 100 bp DNA Ladder Marker (Trans DNA Marker, TransGen Biotech, Inc., Beijing, China); Lane 1–7: genomic DNA of yeast transformants as the template; Lane 8: genomic DNA of G. macrophylla as the template; Lane 9: genomic DNA of control yeast cells treated with ion implantation and incubated in TE buffer without G. macrophylla genomic DNA as the template.
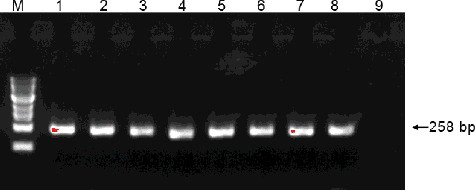
Figure 2. PCR analysis of the SLS gene in G10H-positive yeast transformants. Lane M: 100 bp DNA Ladder Marker (Trans DNA Marker, TransGen Biotech, Inc., Beijing, China); Lane 1: genomic DNA of the control yeast cells treated with ion implantation and incubated in TE buffer without G. macrophylla genomic DNA as the template; Lane 2: genomic DNA of G. macrophylla as the template; Lanes 3–9: genomic DNA of G10H-positive yeast transformants as the template.
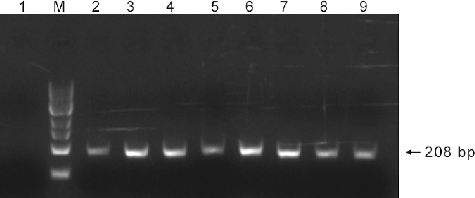
Figure 3. Qualitative screening of the target gentiopicroside-producing transformant using TLC. (A) sample solution of gentiopicroside standard; (B) culture supernatants of the control yeast cells treated with ion implantation and incubated in TE buffer without G. macrophylla genomic DNA; (C) culture supernatants of strain DL67.

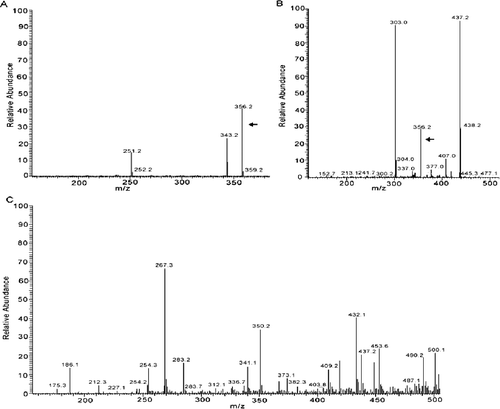
Figure 4. HPLC-MS spectra of the sample from authentic gentiopicroside. Sample solution of gentiopicroside standard (A); culture supernatants of strain DL67 (B); culture supernatants of the control yeast cells treated with ion implantation and incubated with TE buffer without G. macrophylla genomic DNA (C).