Figures & data
Table 1. Primers used in this study.
Figure 1. Optimization of time (A,B) and temperature (C,D) for the expression of Xyn27. (A) SDS-PAGE analysis with different induction time: (M) protein marker (Thermo Fisher Scientific, Shanghai, China), (1) 12 h, (2) 24 h, (3) 36 h, (4) 48 h, (5) 60 h, (6) 72 h; (B) enzyme activity assays under different induction time. (C) SDS-PAGE analysis with different induction temperature: (M) protein marker, (1) 20 °C, (2) 25 °C, (3) 30 °C; (D) enzyme activity assays under different induction temperatures. Note: The highest activity at 60 h was used as 100% (B). The experiments were carried out three times, and each experiment included triplicates. Standard errors of the means (±SEM) were calculated from three independent experiments.
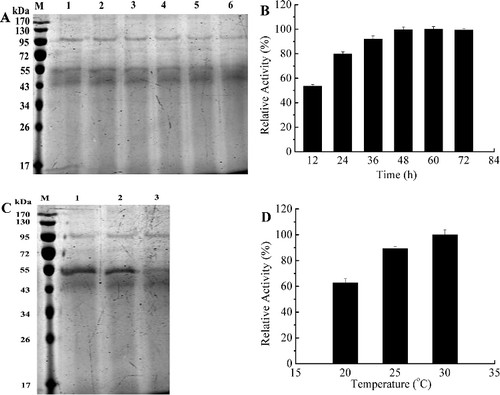
Figure 2. SDS-PAGE analysis of purified recombinant Xyn27. Lanes: M, protein marker (Thermo Fisher Scientific); (1) unpurified recombinant Xyn27; (2) purified recombinant Xyn27.
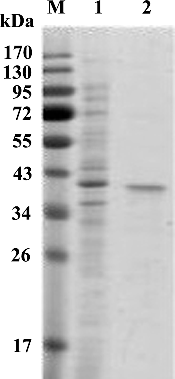
Figure 3. Ezymatic characterization of recombinant Xyn27. Effect of pH (A) on xylanase activity at 35 °C; effect of temperature (B) on xylanase activity at pH 7.0; pH stability (C) at 37 °C for 1 h in buffers of pH 3.0 to 10.0; thermostability (D) at 45 °C, 55 °C and 60 °C. Note: The enzyme activity at pH 7.0 (A), at 35 °C (B) or without any treatment (C,D) was taken as 100%. The experiments were carried out three times, and each experiment included triplicates. Standard errors of the means (±SEM) were calculated from three independent experiments.
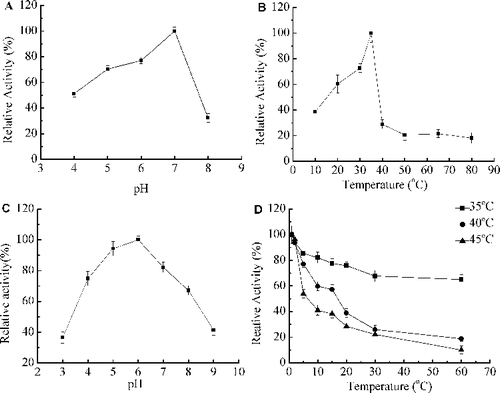
Figure 4. Sequence alignment of Xyn27 with other mesophilic and thermophilic GH10 xylanases. Note: Identical residues are shaded in black; conserved residues are shaded in gray; putative catalytic residues are marked by a triangle. The dotted-line frames indicate the designed degenerate primers sequence. The boldface A, G, R, K are the putative amino-acid sites responsible for the cold-active property of Xyn27.

Table 2. Factors affecting the stability and flexibility of Xyn27 compared with other mesophilic or thermophilic counterparts.
Figure 5. Effects of metal ions and chemicals on the activity of Xyn27. Note: The experiments were carried out three times, and each experiment included triplicates. Standard errors of the means (±SEM) were calculated from three independent experiments.
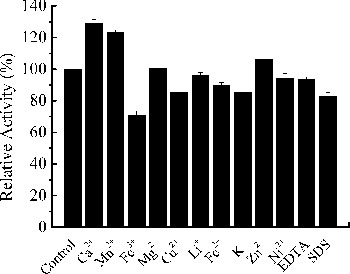
Table 3. Kinetic parameters of recombinant Xyn27 and other cold-active xylanases.