Figures & data
Figure 1. Inductive expression and identification of the recombinant His6-tagged TNF30 in E. coli. (A) SDS-PAGE detection of the products. (B) Western bolt analysis of the products using anti-His6 antibodies.
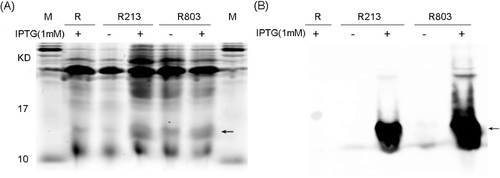
Figure 2. Optimisation of the culture medium for the soluble expression of the recombinant His6-tagged TNF30. (A) SDS-PAGE analysis of the products fermented in different media. (B) Relative quantification of the recombinant His6-tagged TNF30 fermented in different media.
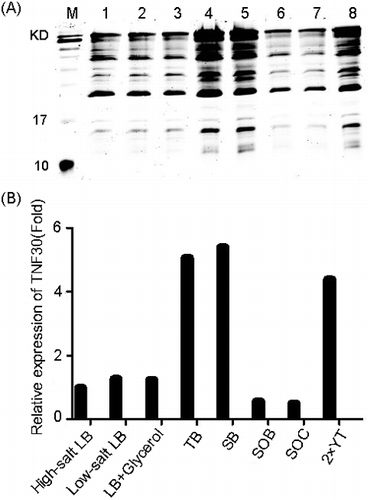
Figure 3. Purification of the recombinant His6-tagged TNF30. Lane M: protein maker (Protein Pre-stained Marker, Thermos); Lane1: supernatant of the recombinant cell lysate; Lane 2: flow-through; Lanes 3–8: gradient elution with imidazole at concentration of 20, 40, 70, 130, 250 and 500 mmol/L, respectively; Lane9: cell lysate supernatant of E. coli Rosetta (DE3) harbouring a pET28a empty plasmid.
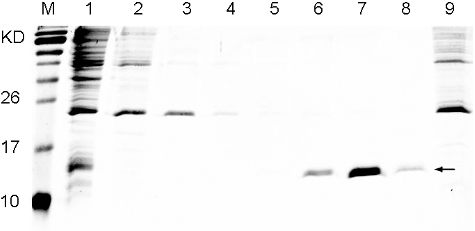
Figure 4. Affinity assay of recombinant TNF30 with TNFα. (A) Products of strains transformed with the empty vector. (B) Recombinant products of strains harbouring His6-fused TNF30.
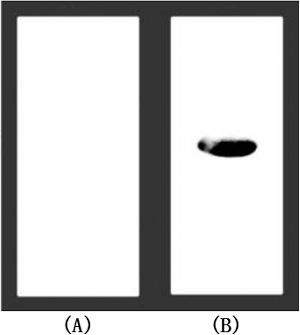
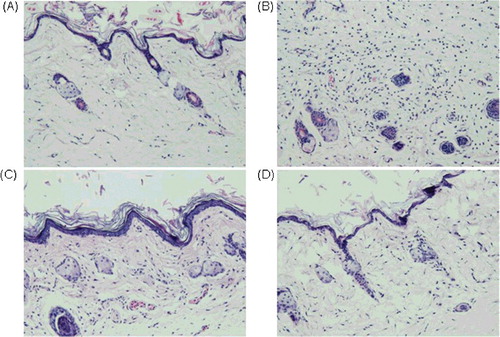
Table 1. Anti-inflammation activity of TNF30 in the carrageenan-induced mice paw oedema model (X±SD; n = 8).