Figures & data
Table 1. Immobilization yield, activity yield, and immobilization efficiency of immobilized enzyme.
Figure 1. Effect of reaction pH on relative activity (%) of free α-amylase (FE) and α-amylase immobilized on AFCCLPANIMg composite (IE). Reaction conditions: Temperature 40 °C, starch concentration 1% w/v, reaction time 20 min.
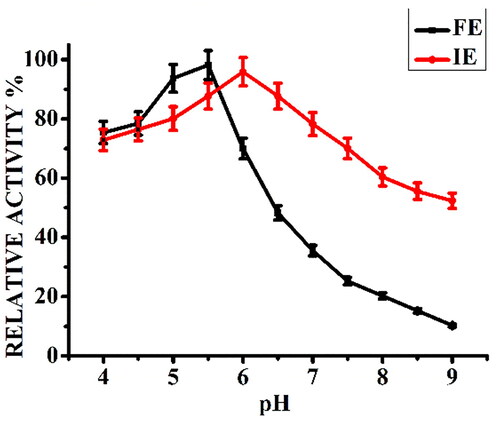
Figure 2. Effect of reaction temperature on relative activity (%) of free α-amylase (FE) and α-amylase immobilized on AFCCLPANIMg composite (IE). Reaction conditions: optimum pH, starch concentration 1% w/v, reaction time 20 min.
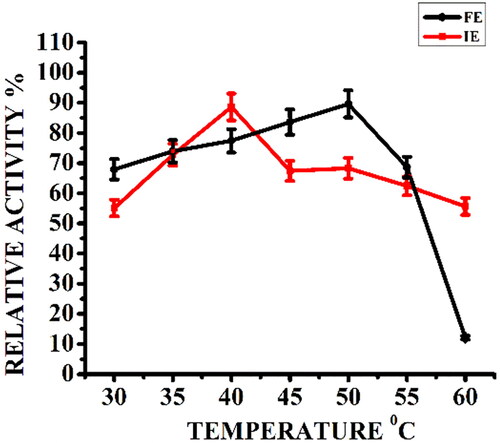
Figure 3. Arrhenius plot for free α-amylase (FE) and α-amylase immobilized on AFCCLPANIMg composite (IE). Reaction conditions: optimum pH, starch concentration 1% w/v, reaction time 20 min.
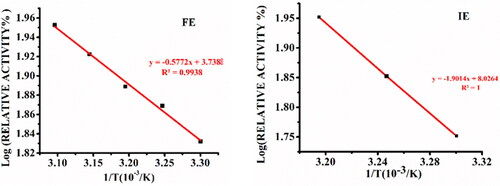
Table 2. Q10 of free enzyme and immobilized enzyme.
Figure 4. Lineweaver Burk plot for free α-amylase (FE) and α-amylase immobilized on AFCCLPANIMg composite (IE). S0 is the initial substrate concentration and Vo is the reaction rate. Reaction conditions: optimum pH, temperature 40 °C, reaction time 20 min.
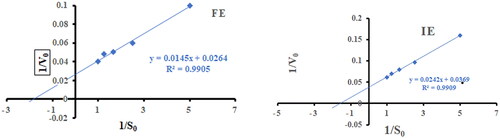
Table 3. Comparison of kinetic parameters of free and immobilized enzyme.
Figure 5. Thermal stability of free α-amylase (FE) and α-amylase immobilized on AFCCLPANIMg composite (IE). The enzymes were incubated at various temperatures from 30 to 70 °C for 1 h in the absence of substrate (a). The enzymes were incubated at optimum temperature for various time from 0-120 min in the absence of substrate (b). In both cases, Reaction conditions: optimum pH, temperature 40 °C, time 20 min, starch concentration 1% w/v.
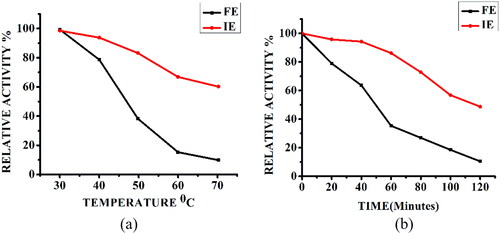
Figure 6. Thermal stability of free α-amylase (FE) and α-amylase immobilized on AFCCLPANIMg composite (IE) by incubating the enzyme at 50 °C, 60°Cand 70 °C for various preincubation time upto120 minutes in the absence of substrate. In both cases, Reaction conditions for activity determination: optimum pH, temperature 40 °C, time 20 min, starch concentration 1% w/v.
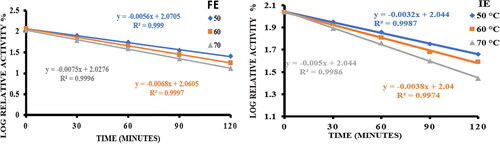
Figure 7. Temperature dependence of the decimal reduction of free α-amylase (FE) and α-amylase immobilized on AFCCLPANIMg composite (IE). Reaction conditions: optimum pH, starch concentration 1% w/v, reaction time 20 min.
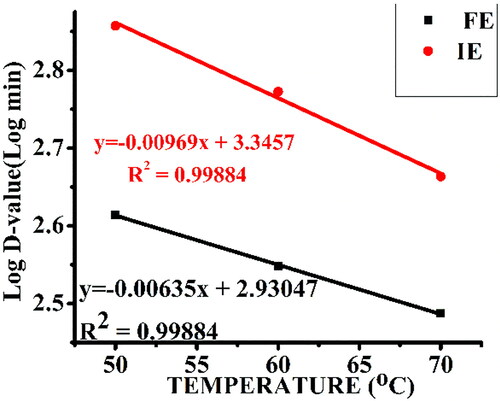
Figure 8. Arrhenius plot of thermal deactivation of free α-amylase (FE) and α-amylase immobilized on AFCCLPANIMg composite (IE). Reaction conditions: optimum pH, starch concentration 1% w/v, reaction time 20 min.
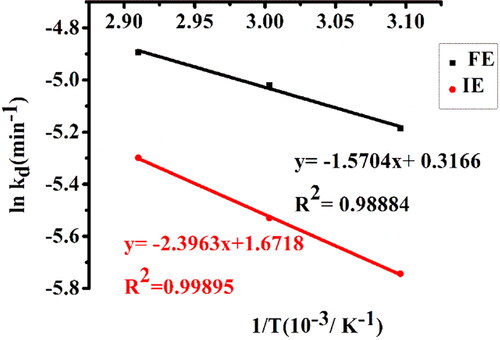
Table 4. Kinetic parameters for thermal deactivation of free enzyme and enzyme immobilized on AFCCLPANIMg composite.
Table 5. Thermodynamic parameters for thermal deactivation of free enzyme and enzyme immobilized on AFCCLPANIMg composite.
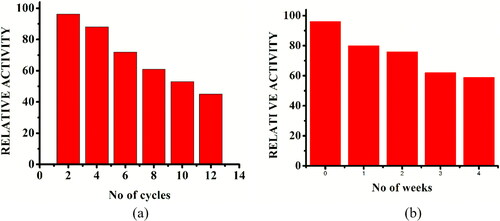
Figure 9. Reusability (a) and storage stability (b) of immobilized inzyme. (a) Reusability of immobilized enzyme for 12 reaction cycles; Reaction conditions: optimum pH, temperature 40 °C, starch concentration 1% w/v, reaction time 20 min; the relative activity of the first run was taken as 100%. (b) Storage stability of immobilized enzyme (stored at 4 °C) for four weeks; Reaction conditions: optimum pH, temperature 40 °C, starch concentration 1% w/v, reaction time 20 min; the relative activity of the initial week was taken as 100%.