Figures & data
Table 1. Toxicity (cell viability) and cell protection by DEAG and Acyclovir (ACV) added simultaneously with and 1 h after the inoculation of the MDBK cell monolayer with SvHA1 (F strain) and SvHA2 (DD strain).
Figure 1. Antiviral activity of DEAG according to an MTT-based colorimetric assay. Virus replication inhibition when DEAG was added simultaneously with the inoculation of a MDBK cell monolayer with SvHA1 (F strain) (![]()
 ); of ACV added simultaneously with the inoculation of an MDBK cell monolayer with SvHA1 (F strain) (
); of ACV added simultaneously with the inoculation of an MDBK cell monolayer with SvHA1 (F strain) ( ), with HuAHV2 (DD strain) (
), with HuAHV2 (DD strain) ( ), with SvHA2 (DD strain) (
), with SvHA2 (DD strain) (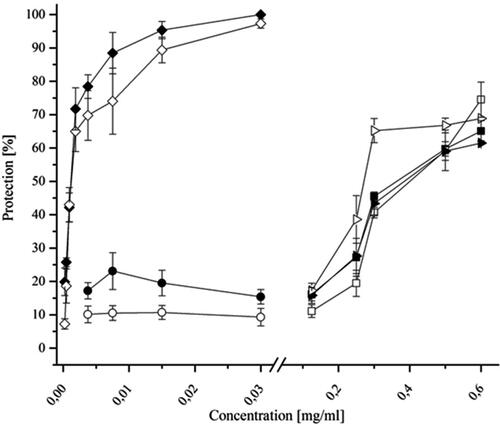
Figure 2. Combined effects of acyclovir and DEAG on the replication of SvHA type 1 in MDBK cells. Acyclovir and DEAG were added 1 h after inoculation of the virus into the cell monolayer, and the inhibition % was determined following incubation for 120 h.
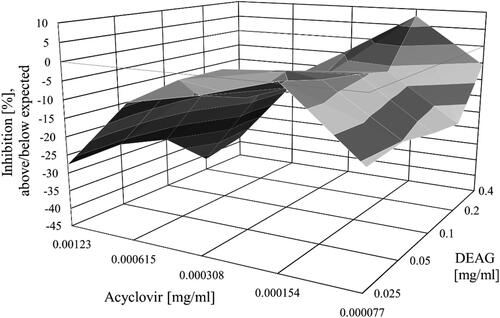
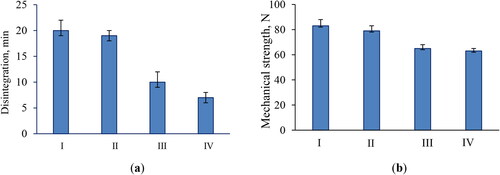
Table 2. Composition of the model tablets (mg/per tablet).
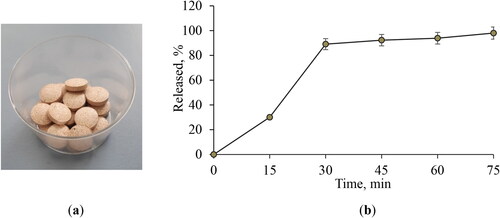
Figure 4. Appearance of the model tablets IV (a) and in vitro release profile of the active compound from the developed tablets (b). Data are mean values ± SD from 3 replicates. Model tablets IV: 150 md DEAG, 43 mg microcrystalline cellulose, 150 mg lactose monohydrate, 5.25 talc and 1.75 magnesium stearate.
Supplemental Material
Download PDF (798.7 KB)Data availability statement
The experimental data supporting this study are freely available from the corresponding author (I.K.) upon reasonable request.