Figures & data
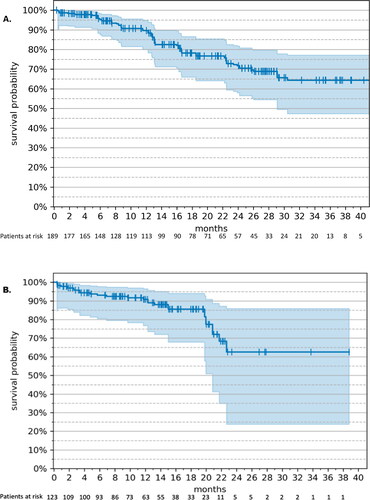
Figure 1. Progression-free survival – RWE cohort vs. MONALEESA-2 (17). (A) Kaplan–Meier estimates. The tick marks represent censored patients; the shaded area represents 95% CI. (B) Weight-adjusted hazard ratio, ribociclib + letrozole (n = 189) vs. letrozole monotherapy (n = 118). Drug: ribocilib + letrozole; Baseline: letrozole monotherapy; ECOG: Eastern Cooperative Oncology Group; ER: estrogen receptor; PR: progesterone receptor; +: positive; −: negative.
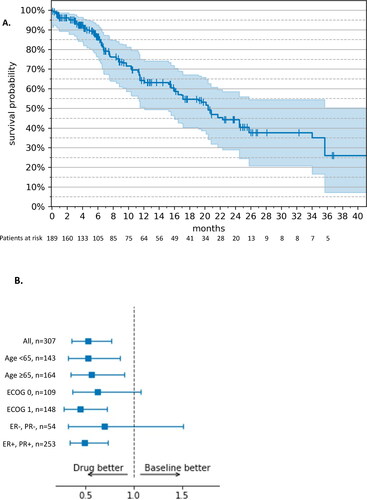
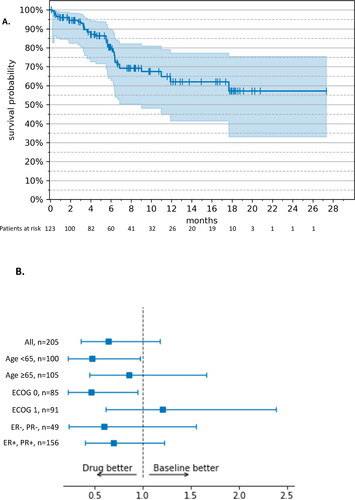
Figure 2. Progression-free survival – RWE cohort vs. MONALEESA-3 (19). (A) Kaplan–Meier estimates. The tick marks represent censored patients; the shaded area represents 95% CI. (B) Weight-adjusted hazard ratio, ribociclib + fulvestrant (n = 123) vs. fulvestrant monotherapy (n = 82). Drug: ribocilib + fulvestrant; Baseline: fulvestrant monotherapy; ECOG: Eastern Cooperative Oncology Group; ER: estrogen receptor; PR: progesterone receptor; +: positive; −: negative.
Supplemental Material
Download ()Data availability statement
Qualified researchers may obtain anonymized data from this study from the corresponding author upon reasonable request.