Figures & data
Figure 1. Verification of ker gene encoding the keratinase enzyme from B. subtilis ES5. Lane 1: control (double distilled water); lane 2: keratinase coding gene (1100 bp); lane 3: DNA ladder (1 kb plus) (thermo fisher scientific, LT-02241 Vilnius, Lithuania, catalog no. 10101240. https://lt.fishersci.com).
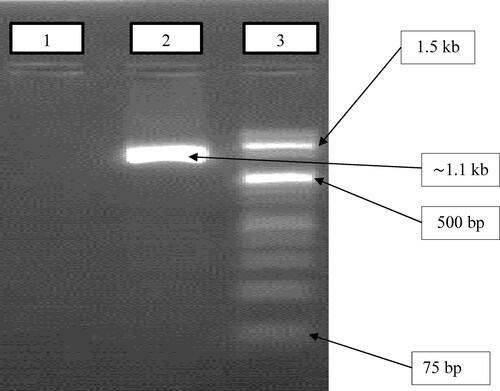
Figure 2. Effect of temperature and pH ranges on the activity and stability of crude keratinase. (A) The optimal temperature for B. subtilis ES5 keratinase activity. Keratinase activity was evaluated by incubation for 30 min at a temperature range of 30–65. (B) Thermal stability of B. subtilis ES5 keratinase. The assay was performed by preincubating the crude keratinase at 50–80
using a water bath for various incubation durations. The activity of the non-heated enzyme was considered 100%. (C) Optimal pH for B. subtilis ES5 keratinase activity. The crude keratinase was incubated in different buffer solutions from pH 5 to 10 using sodium acetate (pH 5–6), phosphate (pH 7–8), and carbonate-bicarbonate (pH 9–12) buffers. (D) pH stability of B. subtilis ES5 keratinase. Keratinase activity was determined by preincubating the crude enzyme at different pH ranges (8–12) for 20, 40, 60, 80, 100, and 120 min using the same buffer systems used above. All the assays were carried out under standard assay conditions. Reported data are the average of triplicates with the standard deviation (± SD).
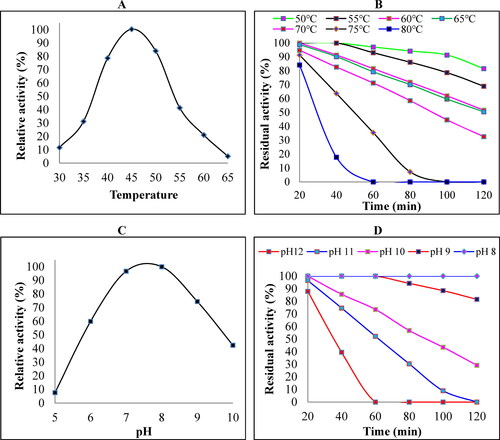
Figure 3. Effects of different metal ions and NaCl concentrations on keratinase activity. The metal ion stability of crude keratinase from B. subtilis ES5 was tested by incubating the enzyme with different concentrations of metal ions for 2 h after adding the substrate. Keratinase activity in the absence of metal ions was taken as 100%, and residual activity was calculated. All results are the mean of triplicate experiments with the standard deviation (± SD) presented as error bars, which differ significantly at p ≤ 0.05 (A). In addition, the stability of the enzyme toward NaCl was evaluated by preincubation with different salt concentrations and revealed that the enzyme is halotolerant (B).
Table 1. Effect of solvents and reducing agents on crude keratinase from B. subtilis ES5.
Figure 4. Dehairing of goat, sheep, and cattle skins/hides with crude keratinase from B. subtilis ES5. Skins/hide without keratinase treatment (A) and skins/hide treated with keratinase (B). The dehaired pelt showed elastic qualities.

Figure 5. Enzymatic-based (A) and conventional (B) dehairing of goat, cattle, and sheep hides/skins. Enzymatic-based dehairing provides a hair-saving method with a white appearance that is more flexible and smoother with an undamaged grain structure because of removal of the epidermis (a), while lime-sulfide dissolves the hair and exhibits a harder grain surface with deposition of foreign particles. The pelt is hardened and less flexible (B).

Figure 6. Finished leather crust. The crust was dehaired by crude keratinase (A) and by lime-sulfide (B). Both enzyme and lime-sulfide dehaired skin pieces were processed to final leather at Bahir Dar Tannery Industry.

Table 2. Physicochemical characterization effluents from dehairing via enzymatic (B. subtilis ES5 keratinase) and conventional approach.
Supplemental Material
Download PDF (484.7 KB)Availability of data
The data that support the findings reported in this study are available from the corresponding author [G.A.] upon reasonable request.
