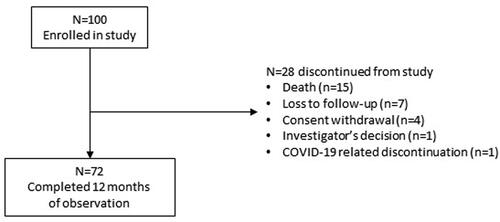
Figures & data
Table 1. Tumor diagnosis and tumor characteristics at baseline.
Table 2. Skeletal-related events and symptomatic skeletal-related events over time.
Figure 2. Time from first denosumab dose to non-persistence with denosumab. LFUP, loss to follow-up; LOCP, last observation carried forward. Calculated from the first dose within the 6-month retrospective period. Persistence was assessed for the 60-day gap. LOCF approach: the loss of persistence was at the last dose before the loss to follow-up + the 60-day gap). LFUP censored approach: patients were considered persistent by the date of loss to follow-up and then they were censored.
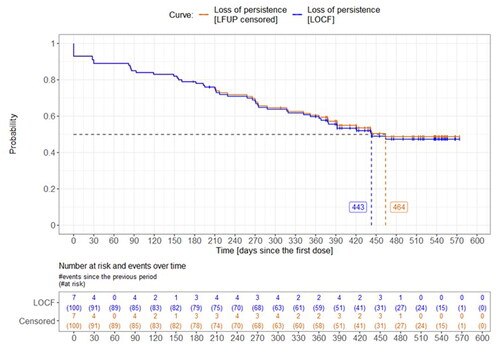
Table 3. Summary of denosumab-related adverse drug reactions (by MedDRA classification).
Supplemental Material
Download PDF (1.2 MB)Data availability statement
Qualified researchers may request data from Amgen clinical studies. Complete data are available at the following: https://wwwext.amgen.com/about/how-we-operate/policies-practices-and-disclosures/ethical-research/clinical-data-transparency-practices/clinical-trial-data-sharing-request.