Figures & data
Figure 1. 2A4 bound to soluble and insoluble LC aggregates from ALMC cells. (A, B) Particles in the soluble LC fraction were analyzed using atomic force microscopy. Small (red arrow), medium (yellow arrow) and large (green arrow) globules are present, alongside apparent short fibrils (orange arrow). (C) Principle of the sandwich MSD electrochemiluminescence assay developed to detect LC aggregates. (D) 2A4 specifically binds ALMC protein that is aggregated. (E) After fractionation by centrifugation, both soluble and insoluble fractions exhibited strong 2A4 reactivity. Values represent the mean ± SEM of results from three optical fields (B) or three experimental replicates (D, E).
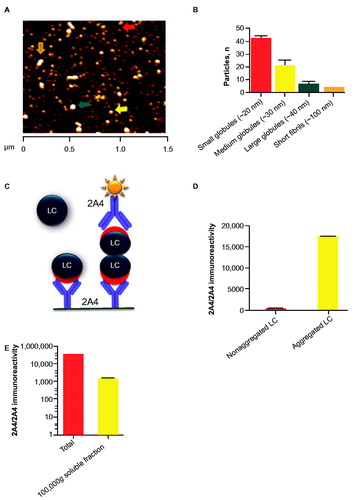
Figure 2. 2A4 binds to both soluble and insoluble aggregates in various organs of patients with AL amyloidosis. Fresh-frozen samples from 10 patients (representing both λ and κ LC amyloid) were assessed using the MSD electrochemiluminescence assay. Strong reactivity to 2A4 was observed in 19 of 20 crude fractions and 6 of 19 soluble fractions studied. Values represent the relative levels of LC aggregates measured as electrochemiluminescence signal.
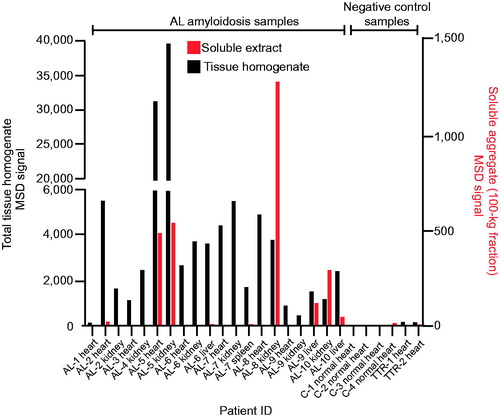
Table 1. Summary of 2A4 immunoreactivity to AL amyloidosis patient tissue.
Figure 3. Size exclusion chromatography and 2A4 reactivity to soluble LC aggregates extracted from the heart of a patient with AL amyloidosis. A wide range of soluble LC aggregates can be immunodepleted by 2A4. Values represent electrochemiluminescence signal.
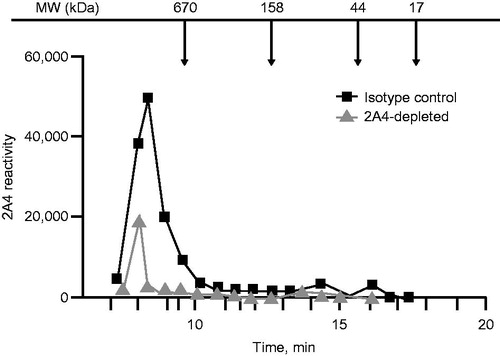
Figure 4. 2A4 specifically immunolabels cardiac and renal λ and κ FLC deposits from patients with light chain (AL) amyloidosis. (A–C) Cardiac 2A4 immunolabeling appears diffusely pericellular, intercalating the fibers of the myocardium. Immunoreactivity overlaps predominantly with ThioT labeling, but amorphous deposits (ThioT-negative) also co-label with the antibody. (D–F) 2A4 strongly decorated the glomeruli and renal vasculature and co-localized with λ and κ FLC deposits (G–L). (M–O) 2A4 immunoreactivity was reduced in fresh-frozen cardiac samples after brief (1 min) post-fixation with formalin, whereas ThioT staining was unaffected. (P-R) 2A4 preabsorptions with aggregated LC proteins, but not monomeric LC, attenuated 2A4 immunoreactivity in cardiac tissue. +Aggr. FLC, aggregated free light chain; Thio T, thioflavin T. A–C, G–L: scale bars = 500 μm; D–F, P–R: scale bars = 250 μm; M–O: scale bars = 100 μm.
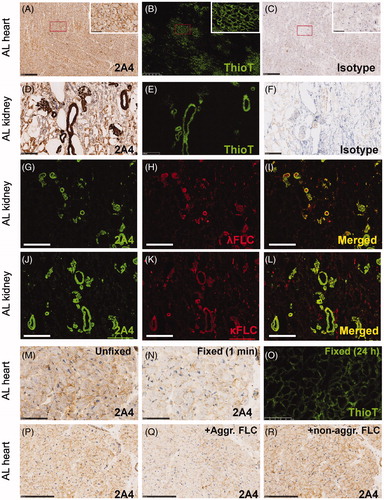
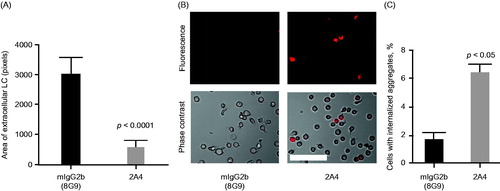
Figure 5. 2A4 promotes phagocytosis of AL amyloidosis heart extracts by a human macrophage cell line in vitro. (A) 2A4 promoted clearance of the extracellular AL heart deposits by THP-1 macrophages. Clearance was measured by area of extracellular LC protein deposits, immunostained for λ LC, remaining after 24 h and was significantly increased by 2A4 treatment (gray bar) compared with an isotype control antibody (black bar). Data are presented as mean ± SEM (n = 3). (B, C) 2A4 induced internalization of pHrodo-conjugated AL heart deposits by THP-1 cells, visualized as an increase in intracellular pHrodo-fluorescence shown in a representative bright-field overlay (20×; B) or quantified as the percentage of THP-1 cells with elevated relative mean fluorescence intensity (C), which represents internalized pHrodo-labeled aggregates. Values represent mean ± SEM of 60 optical fields per group, pooled from three independent wells (Figure 5A) or of three independent flow cytometry runs with approximately 1000 cells per run (Figure 5C). Statistical differences were determined by 2-tailed Student t test. Scale bar = 100 μm.