Figures & data
Figure 1. Interventional studies in patients with ATTR-PN. Summary of clinical studies in patients with ATTR-PN that were pooled in this analysis. Tafamidis dose was 20 mg once daily in each study. NCT00791492 is an extension study of NCT00409175. NCT00925002 is an extension study of NCT00791492 and NCT00630864. aStudies with duration “until available” are ongoing until tafamidis is commercially available for the patient in patient’s country. ATTR-PN: transthyretin amyloid polyneuropathy.
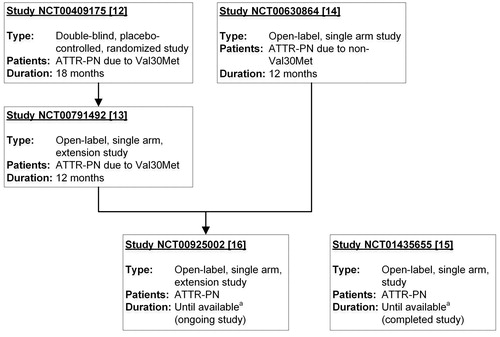
Table 1. Demographic and baseline characteristics in all completed and ongoing studies in patients with ATTR-PN.
Table 2. Duration of exposure to tafamidis in completed and ongoing studies in patients with ATTR-PN and in the non-interventional THAOS registry (NCT00628745).
Table 3. Summary of most commonTable Footnotea medical history in all completed and ongoing studies in patients with ATTR-PN.
Table 4. Summary of most common TEAEs in all completed and ongoing studies in patients with ATTR-PN.
Table 5. Summary of most common TEAEs in the non-interventional THAOS registry (NCT00628745).
Table 6. Summary of most common TEAEs reported in the spontaneous safety reporting database.
Data availability
Upon request, and subject to certain criteria, conditions and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines and medical devices (1) for indications that have been approved in the USA and/or EU or (2) in programmes that have been terminated (i.e. development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
