Figures & data
Figure 1. Patient disposition. *Modified intent-to-treat population: all patients who were randomized and received at least one dose of the study drug. †Numbers of discontinuations to the end of 18 months. One patient in each treatment group discontinued due to suspected or confirmed diagnosis of COVID-19 or due to the impact of the global COVID-19 pandemic, reported in addition to the primary reason for treatment discontinuation. There were two deaths due to COVID-19, one in each treatment arm.
Table 1. Baseline demographics and clinical characteristics.
Figure 2. Key efficacy and pharmacodynamic endpoints assessing the effect of vutrisiran. LS mean change from baseline in (A) mNIS+7, (B) Norfolk QOL-DN, and (C) percent change from baseline in serum TTR levels with vutrisiran and patisiran through 18 months of the HELIOS-A study. The mNIS+7 and Norfolk QOL-DN data were calculated using the modified intent-to-treat population; the reduction in serum TTR was calculated using the TTR per-protocol population. *Higher scores of mNIS+7 indicate more neuropathy impairment (range, 0–304). At baseline, the mean (±SD) mNIS+7 was 60.6 (36.0) in the vutrisiran group and 74.6 (37.0) in the external placebo group. Data at 9 months are from the ANCOVA/multiple imputation model and data at 18 months are from the MMRM model. †Higher scores of Norfolk QOL-DN indicate worse quality of life (range, –4 to 136). At baseline, the mean (±SD) Norfolk QOL-DN score was 47.1 (26.3) in the vutrisiran group and 55.5 (24.3) in the external placebo group. Data at 9 months are from the ANCOVA/multiple imputation model and data at 18 months are from the MMRM model. ANCOVA: analysis of covariance; CI: confidence interval; LS: least squares; LSMD: least squares mean difference; MMRM: mixed-effects model for repeated measures; mNIS+7: modified Neuropathy Impairment Score +7; Norfolk QOL-DN: Norfolk Quality of Life-Diabetic Neuropathy; SD: standard deviation; SE: standard error; TTR: transthyretin.
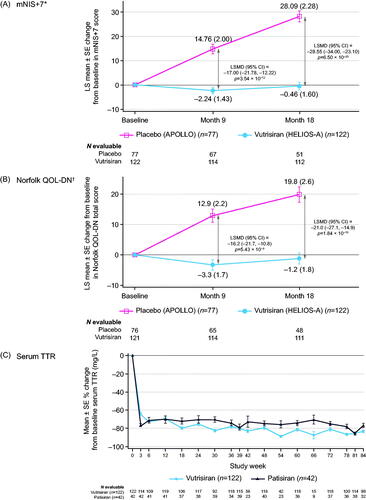
Table 2. Select secondary and exploratory endpoints in the mITT population.
Table 3. Summary of adverse events.
Supplemental Material
Download MS Word (31.8 KB)Supplemental Material
Download MS Word (1.9 MB)Data availability statement
De-identified individual participant data that support these results will be made available in a secure-access environment 12 months after study completion and when the product and indication have been approved for no less than 12 months in the US and the EU. Access will be provided contingent upon the approval of a research proposal and the execution of a data sharing agreement. Requests for access to data can be submitted via the website www.vivli.org.
