Figures & data
Figure 1. Tafamidis is a WT TTR kinetic stabiliser in pooled human cerebrospinal fluid (CSF) from healthy donors. (A) Schematic of the steps involved in TTR tetramer dissociation and reassociation of protomers associated with subunit exchange between untagged endogenous WT TTR subunits (blue squares) and (His)6-FT2-C10A TTR reporter subunits (yellow squares) Note that only one of three possible subunit arrangements is shown for the 2:2 tetramer. (B) The kinetics of TTR subunit exchange in CSF over nine days between endogenous WT TTR (∼203 nM) and (His)6-FT2-C10A reporter TTR (200 nM) as a function of increasing tafamidis concentration. (C) Average kinetic rate constants obtained from fitting the data, revealing dose-dependent stabilisation of WT TTR in CSF with increasing tafamidis concentration (D) Ex vivo incubation of tafamidis in healthy pooled CSF shows that decreasing the [TTR tetramer]/[tafamidis] ratio increases stability of endogenous WT TTR in human CSF. The colours in all panels correspond to the key shown in panel B.
![Figure 1. Tafamidis is a WT TTR kinetic stabiliser in pooled human cerebrospinal fluid (CSF) from healthy donors. (A) Schematic of the steps involved in TTR tetramer dissociation and reassociation of protomers associated with subunit exchange between untagged endogenous WT TTR subunits (blue squares) and (His)6-FT2-C10A TTR reporter subunits (yellow squares) Note that only one of three possible subunit arrangements is shown for the 2:2 tetramer. (B) The kinetics of TTR subunit exchange in CSF over nine days between endogenous WT TTR (∼203 nM) and (His)6-FT2-C10A reporter TTR (200 nM) as a function of increasing tafamidis concentration. (C) Average kinetic rate constants obtained from fitting the data, revealing dose-dependent stabilisation of WT TTR in CSF with increasing tafamidis concentration (D) Ex vivo incubation of tafamidis in healthy pooled CSF shows that decreasing the [TTR tetramer]/[tafamidis] ratio increases stability of endogenous WT TTR in human CSF. The colours in all panels correspond to the key shown in panel B.](/cms/asset/4ce21cda-d114-4a75-bbf5-8c57c217b0f5/iamy_a_2167595_f0001_c.jpg)
Table 1. Average kinetic rate constants of TTR subunit exchange in CSF.
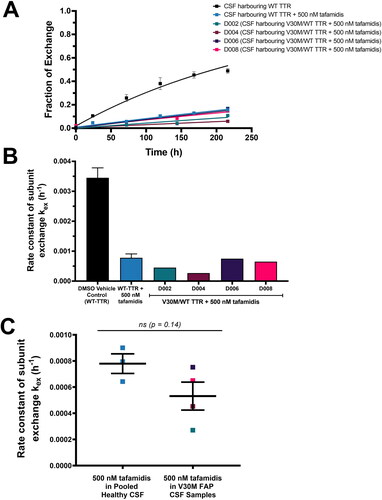
Figure 2. Tafamidis is an effective kinetic stabiliser of the TTR tetramers comprising human CSF from four heterozygous V30M/WT TTR familial amyloid polyneuropathy (FAP) patients. (A) Four subunit exchange time courses between endogenous V30M/WT TTR and (His)6-FT2-C10A-V30M TTR reporter added to human CSF derived from the four heterozygous V30M/WT CSF patients whose samples are identified as D002, D004, D006 and D008 (coloured traces; ≈ 266 nM tetrameric TTR) in the presence of 500 nM tafamidis. The black time course at the top depicts the subunit exchange kinetics of WT TTR (without added tafamidis), whereas the blue time course partially obscured by the coloured traces shows the subunit exchange kinetics of WT TTR with 500 nM tafamidis added. Both of these subunit exchange-based TTR kinetic stability measurements were performed using (His)6-FT2-C10A TTR as the reporter. (B) Calculated subunit exchange rate constants (C) Comparison of the subunit exchange rates extracted from the time courses shows no significant difference between the TTR kinetic stability afforded by 500 nM tafamidis in CSF comprising WT TTR and 500 nM tafamidis in CSF harbouring V30M/WT tetramers from polyneuropathy patient CSF. The colours in all panels correspond to the key shown in panel A.