Figures & data
Figure 1 Two pathways of NO generation under normoxia or hypoxia and the sequential signaling pathway afterward. NOS catalyzes the oxidation of l-arginine to l-citrulline in the presence of O2 (left). Nitrite reductase catalyzes NO2− reduction to generate NO. Then NO activates sGC to produce cGMP, which triggers smooth muscle relaxation.
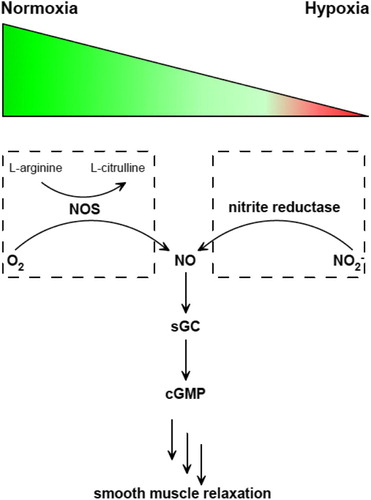
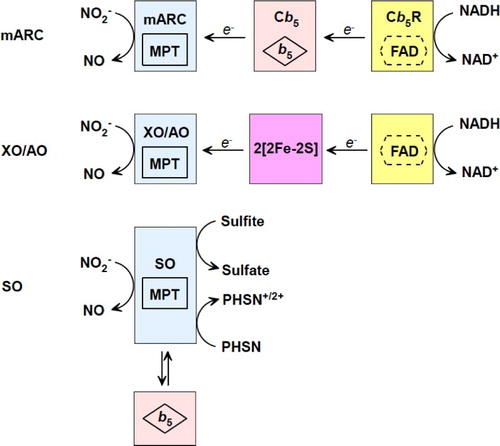
Table 1 UV–vis of active centers in XO, AO, SO, mARC, cyt b5, and cyt b5R.
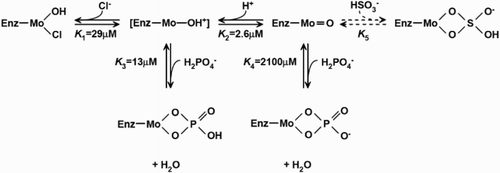
Figure 2 Proposed low pH, high pH, bisulfite, and phosphate-binding to the Mo domain of SO. Values of the equilibrium were the ones which gave the best fit to the experimental data.