Figures & data
Scheme 1. Possible pathways involved in the photosensitized oxidation of CTQ, CTC, and RSC. S represents the photosensitizer and dCTQ denotes generically to the substrates CTQ, CTC, and RSC.
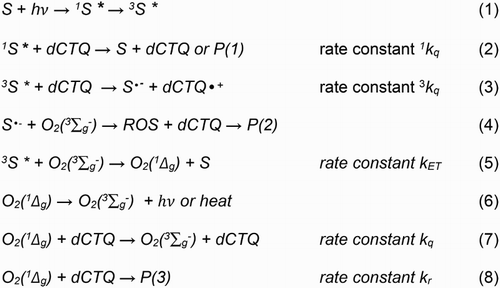
Figure 1. Spectral changes of 0.4 mM CTQ + 0.02 mM Rf vs. 0.02 mM Rf upon visible-light photoirradiation, in buffer pH 7 (main figure) and in buffer pH 9 (insert). Numbers on the spectra represent irradiation time, in seconds.
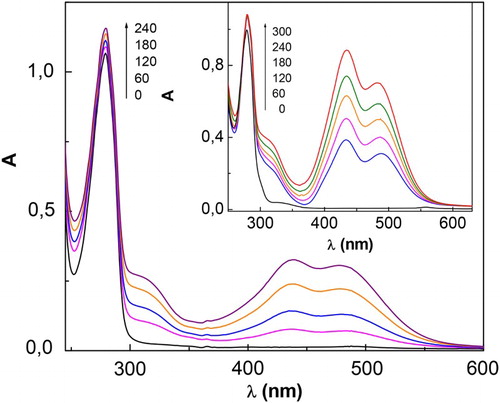
Figure 2. Spectral changes of 0.7 mM RSC + 0.02 mM Rf vs. 0.02 mM Rf, upon visible-light photoirradiation. Insert: spectral changes for 0.4 mM CTC + 0.02 mM Rf vs. 0.02 mM Rf, upon visible-light photoirradiation. Solvent: buffer pH 7. Numbers on the spectra represent irradiation time, in seconds.
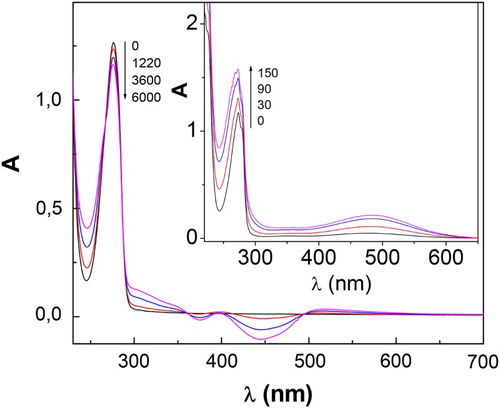
Figure 3. Profiles of oxygen consumption, upon Rf-sensitized photoirradiation (a) CTC, (b) CTQ, and (c) RSC, in buffer pH 7 (main figure) and pH 9 (insert). [dCTQ] = 0.4 mM.
![Figure 3. Profiles of oxygen consumption, upon Rf-sensitized photoirradiation (a) CTC, (b) CTQ, and (c) RSC, in buffer pH 7 (main figure) and pH 9 (insert). [dCTQ] = 0.4 mM.](/cms/asset/9520f804-ec3a-4003-a3db-33f446cd0c52/yrer_a_1240287_f0003_c.jpg)
Table 1 Relative rates of oxygen consumption by CTQ, CTC, and RSC, sensitized by RB (A556 nm = 0.6) and 0.02 mM Rf. Solvents: buffer pH 7 and buffer pH 9.
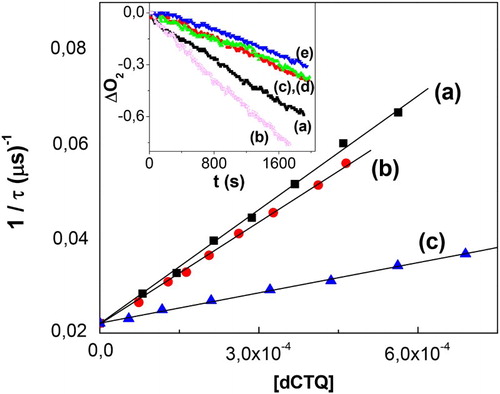
Figure 4. Stern–Volmer plot for the quenching of O2 -phosphorescence emission by dCTQ: (a) CTQ, (b) CTC, and (c) RSC, upon RB-sensitized photoirradiation, in D2O pD 7. Inset: profiles of oxygen consumption, in buffer pH 7, upon Rf-sensitized photoirradiation (a) 0.02 mM Rf + 0.4 mM CTQ; (b) 0.02 mM Rf + 0.4 mM CTQ + 1 mg/100 ml SOD; (c) 0.02 mM Rf + 0.4 mM CTQ + 10 mM NaN3; (d) 0.02 mM Rf + 0.4 mM CTQ + 1 mg/100 ml CAT; (e) 0.02 mM Rf + 0.4 mM CTQ + 1.0 mM D-Mannitol (1.0 mM).