Figures & data
Table 1. Characteristics of vulnerable atherosclerotic plaques and putative underlying processes.
Figure 1. The MPO catalytic cycle. Native MPO reacts with H2O2 to form compound I. Compound I can be converted back to native MPO either by the halogenation cycle or by the two-step one-electron reduction in the peroxidase cycle. Ferrous-MPO and compound III are redox forms of MPO that exist outside the two catalytic cycles.
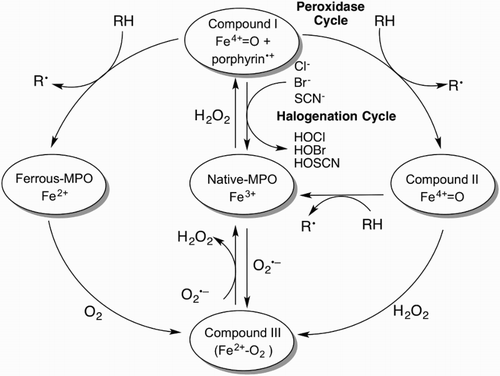
Table 2. Potential pro-atherogenic actions of MPO and putative underlying processes.
Table 3. Potential contributions of HOCl-modified LDL to the development of atherosclerosis.
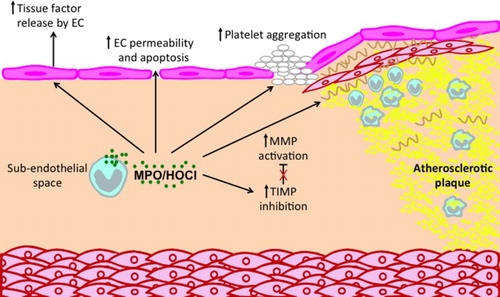
Figure 2. Potential roles of MPO and MPO-derived oxidants in promoting atherosclerotic plaque instability. MPO can affect a number of processes that contribute to plaque instability and possible plaque rupture. MPO released by monocyte/macrophages and neutrophil can activate MMPs and inhibit TIMPs, leading to reduction in ECM, especially in the fibrous cap. In addition, MPO may contribute to a thrombogenic environment by inducing endothelial cells to release tissue factor and by priming of platelet aggregation. Finally, MPO can increase endothelial cell (EC) permeability and apoptosis, thereby increasing the leakiness of the endothelium.