Figures & data
Figure 1. Complex II assembly and its metabolic activity. (A) CII is assembled from four structurally different subunits SDHA-D. SDHA, with covalently bound FAD, requires two assembly factors SDHAF2 and SDHAF4 that assist with SDHA maturation and flavinylation. SDHB contains three iron-sulphur (FeS) clusters, and two additional assembly factors SDHAF1 and SDHAF3 are needed for their insertion. Dimer of SDHA/B is then linked to transmembrane subunits SDHC/D. Under some conditions, an alternative assembly species of CII consisting of SDHA, SDHAF2, and SDHAF4 (described as CIIlow) is stabilized and has an independent biological function. (B) Metabolic activity of the mature CII. CII promotes oxidation of succinate to fumarate. Electrons from succinate are removed by FAD in SDHA and then passed to FeS clusters of SDHB subunit. FeS cofactors transfer the electrons to ubiquinone (CoQ) within the ubiquinone binding (Q) site formed by SDHB, SDHC, and SDHD.
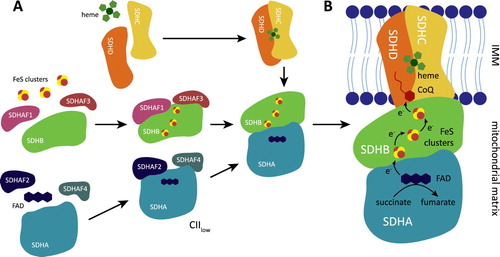
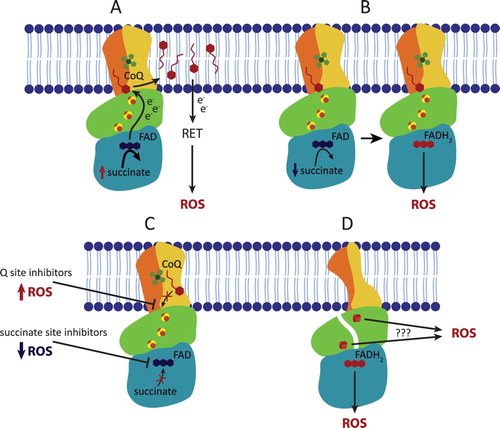
Figure 2. Complex II contributes to ROS production in both physiological and pathophysiological conditions. (A) In the presence of high concentrations of succinate, CII does not produce ROS directly but can contribute to indirect ROS generation via reverse electron transfer (RET) by forcing the electrons onto CI. (B) At lower, physiological succinate concentration, succinate molecule passes the electrons to FAD forming FADH2 which is then able to react with oxygen within the unoccupied succinate binding site, therefore directly forming ROS. (C) The ROS generating ability of reduced FAD is significantly increased when the Q site is blocked by an inhibitor. In contrast, succinate binding site inhibitors block ROS production. (D) Incorrect assembly or damage to CII subunits can induce ROS formation either via reduced FAD or possibly via exposed FeS clusters.