Figures & data
Figure 1. Exogenous H2S relieves blue light-induced retinal degeneration and apoptosis in rats. (A) Eyelid surgery procedure and its effects. (B) Retinal tissue morphology was detected by H&E staining (n = 6 eyes per group). (C) Statistics of whole retinal thickness. (D) Statistics of INL and ONL thickness. (E) Statistics of RPE cell layer thickness. (F) Retinal tissue sections of rats were stained with DAPI and TUNEL to detect apoptosis (n = 3 eyes per group). Ctr, control group. BL, blue light treatment. NS, normal saline. Values are mean ± SD. **p < 0. 01, ***p < 0.001 versus the control group; ##p < 0.01, ###p < 0.001 versus the BL + NS group.
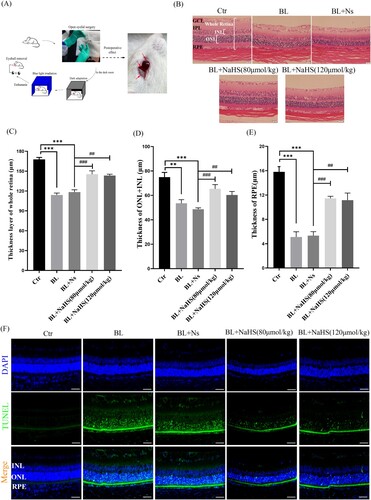
Figure 2. Exogenous H2S inhibits blue light-triggered retinal oxidative stress and ER stress in vivo. (A) ROS in the retina were labeled with the fluorescent probe DHE and detected by a fluorescent microscope (n = 3 eyes per group). (B) Expression of retinal CHOP protein was detected by immunohistochemistry (n = 6 eyes per group). (C) Western blot assay was used to detect the protein levels of GRP78 and CHOP in retinal tissue (n = 3 eyes per group). (D) Statistics of GRP78 protein alteration. (E) Statistics of CHOP protein alteration. Values are mean ± SD. **p < 0. 01, ***p < 0.001 versus the control group; ##p < 0.01, ###p < 0.001 versus the BL + NS group.
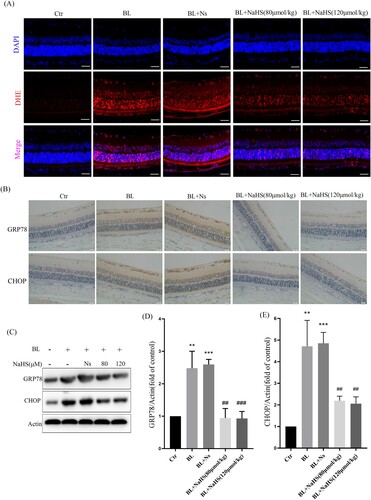
Figure 3. H2S inhibits blue light-triggered oxidative damage in ARPE-19 cells. (A) ARPE-19 Cells were pretreated with NaHS for 1h and then exposed to blue light for 4 h. Cells were stained with DCFH-DA, and intracellular ROS levels were observed by flow cytometry (n = 3). (B) Statistics of intracellular ROS level. (C) ARPE-19 Cells were pretreated with NaHS for 1 h and then exposed to blue light for 4 h, and then cultured for another 24 h. The intracellular SOD activity was detected by the assay kits (n = 3). (D) ARPE-19 cells were exposed to blue light for indicated time, and then cultured for another 24 h. The MDA assay kit used to detect MDA level in cells (n = 3). (E) ARPE-19 Cells were pretreated with NaHS for 1 h and then exposed to blue light for 4 h, and then cultured for another 24 h. The MDA assay kit was used to detect MDA level in cells (n = 3). Values are mean ± SD. *p < 0. 05, ** p < 0. 01, *** p < 0.001 versus the control group; # p < 0.05, ## p < 0.01 versus the BL alone group.
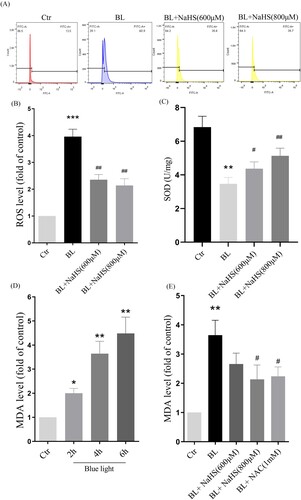
Figure 4. H2S inhibits blue light-induced activation of ER stress-CHOP apoptotic signal in ARPE-19 cells. (A) ARPE-19 cells were exposed to blue light for indicated time, and then cultured for another 24 h. Western blot assay was used to detect the protein levels of GRP78 and CHOP (n = 3). (B) Statistics of GRP78 protein alteration. (C) Statistics of CHOP protein alteration. (D) ARPE-19 Cells were pretreated with NaHS for 1 h and then exposed to blue light for 4 h, and then cultured for another 24 h. Western blot assay was used to detect the protein levels of GRP78 and CHOP (n = 3). (E) Statistics of GRP78 protein alteration. (F) Statistics of CHOP protein alteration. Values are mean ± SD. *p < 0. 05, **p < 0. 01, ***p < 0.001 versus the control group; #p < 0.05, ##p < 0.01 versus the BL alone group.
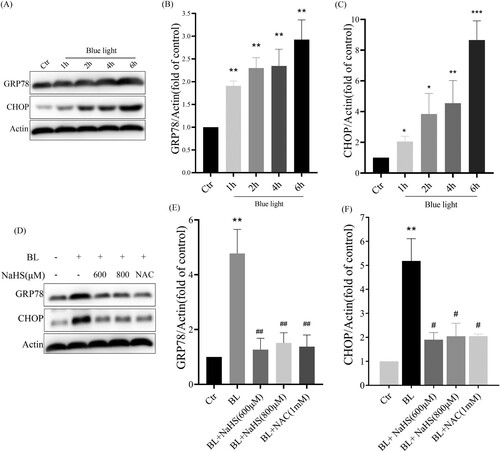
Figure 5. ER stress-CHOP apoptosis signal is involved in blue light-induced apoptosis in ARPE-19 cells. (A) ARPE-19 cells were pretreated with 4-PBA for 2 h and exposed to blue light for 4 h, and then cultured for another 24 h. Western blot assay was used to detect the protein levels of GRP78 and CHOP (n = 3). (B) MTT assay was used to analyze cell viability (n = 3). (C) Apoptosis was detected by flow cytometry with Annexin V-FITC and PI staining (n = 3). (D) Statistics of cell apoptosis. (E) Cell death was analyzed with Hochest33342 and PI staining, and observed under a fluorescent microscope (n = 3). Values are mean ± SD. **p < 0. 01, ***p < 0.001 versus the control group; ## p < 0.01, ###p < 0.001 versus the BL alone group.
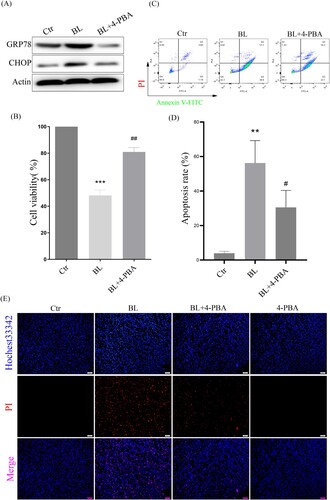
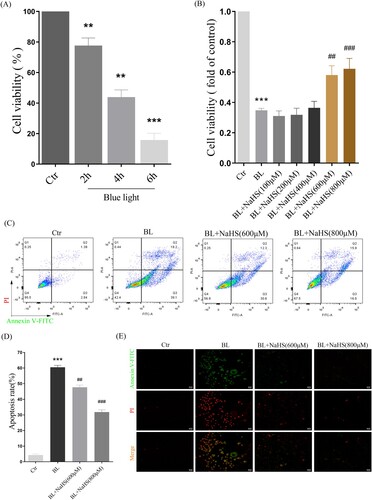
Figure 6. H2S reduces blue light-induced cell proliferation arrest and apoptosis in ARPE-19 cells. (A) ARPE-19 cells were exposed to blue light for indicated time, and then cultured for another 24 h. MTT assay was used to detect the cytotoxicity of blue light exposure (n = 3). (B) ARPE-19 cells were pretreated with NaHS for 1 h and exposed to blue light for 4 h, and then cultured for another 24 h. MTT assay was used to detect cell viability (n = 3). (C) Apoptosis was detected by flow cytometry with Annexin V-FITC and PI staining (n = 3). (D) Statistics of cell apoptosis. (E) Apoptosis was detected by a fluorescent microscope with Annexin V-FITC and PI staining (n = 3). Values are mean ± SD. **p < 0. 01, *** p < 0.001 versus the control group; ## p < 0.01, ### p < 0.001 versus the BL alone group.