Figures & data
Figure 1. US FDA-approved countermeasures for RN threats following the Animal Rule and other promising agents. In 2002, the FDA issued the Animal Rule to expedite the development of medical countermeasures against CBRN threats. Since then, 2 countermeasures (*) have been approved by the FDA following the Animal Rule.
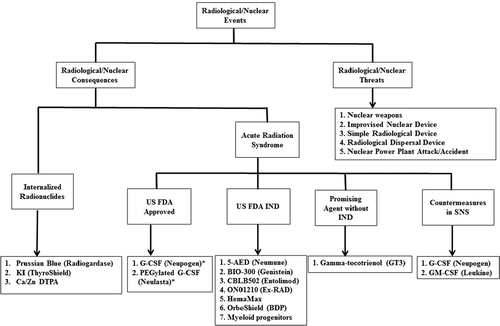
Table 1. Patents of countermeasures for ionizing radiation.
