Figures & data
Figure 1. Structures of aztreonam (1) and selected examples of siderophore-conjugated monocyclic β-lactams from literature. Note that parts of the molecule containing siderophore mimetics are highlighted in blue.
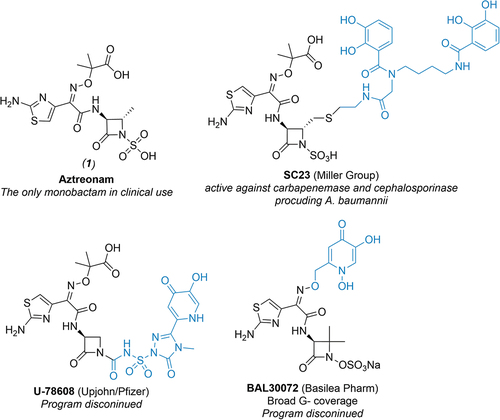
Figure 2. Summary of the present invention and key advantages of the chosen approach. Note that key structural features of aztreonam, including (i) aminothiazoloxime (ATMO) sidechain which enhances Gram-negative activity, (ii) an essential β-lactam ring acylating warhead, (iii) methyl group that improves stability toward β-lactamases, and (iv) N-1 heteroatom activation provided by sulfonic acid are highlighted in orange, violet, cyan, and salmon color, respectively. A site where patent assignees envisaged the installation of siderophore mimetic with a hope to enhance activity against MDR Gram-negative pathogens is shown in a green box.
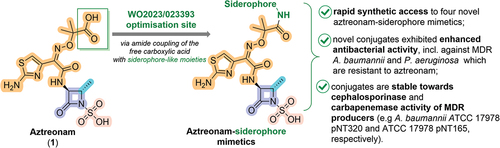
Figure 3. General Markush structure of the new compound series, chemical structure of the control compound, and chemical structures of key novel functionalized monocyclic-β-lactam derivatives from the patent assignees. The siderophore-containing regions are highlighted in green (bis-catechol based ligands).
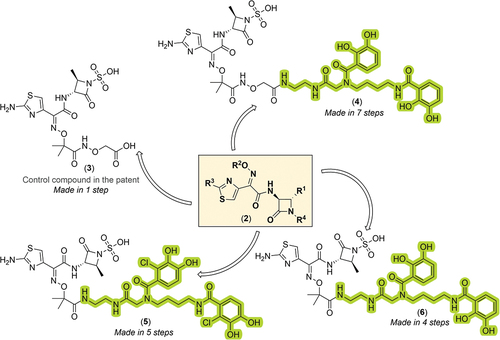
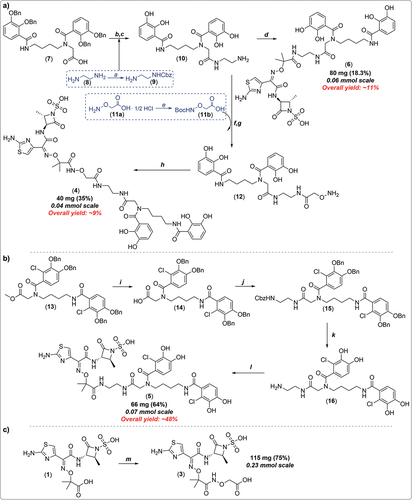
Figure 4. Summary of synthetic strategies reported by the patent assignees required to prepare novel analogues 3–6. reagents and conditions: (a)* benzylchloroformate, CH2Cl2, 0°C, 3.5 h; (b) N-hydroxysuccinimide (NHS), EDC·HCl, DMF, rt, then 9, several hours; (c)* 10% Pd/C, MeOH, H2 balloon, rt, 16 h; (d) 1 in DMF, HBTU, DIPEA, 10 min, rt, then 10 in DMF, rt, 16 h; (e)* Et3N, (Boc)2O, CH2Cl2, 0°C to rt; (f) 11b in DMF, HBTU, DIPEA, 10 min, rt, then 10 in DMF, 16 h; (g)* TFA, CH2Cl2, rt; (h) 1, THF-H2O (50/50, v/v; pH = 4.5), EDC·HCl; (i)* NaOH, THF-H2O (2:1; pH = 4.5), rt, 3 h, then 1N HCl; (j) NHS, EDC·HCl, DMF, rt, 3 h, then benzyl (2-aminoethyl)carbamate (9), DIPEA, rt, 16 h; (k)* 10% Pd/C, MeOH, H2 balloon, rt, 16 h; (l) 1 in DMF, HBTU, DIPEA, 10 min, rt, then 16 in DMF, rt, 16 h; (m) NHS, EDC·HCl, DMF, rt, then 11a, DIPEA, rt, several hours. Note that asterisk (*) denotes product used in the next step without further purification. For multi-step reaction sequences, overall yields (in red) were estimated using the longest linear sequence/available data.