Figures & data
Figure 1. Selectivity of jakinibs for different JAK isoforms and cytokine signaling via JAK/STAT pathways. JAKs are tyrosine kinases that become capable to bind the intracellular portion of several type I and II cytokine receptors upon ligation of the receptor by the cytokine.
A plethora of cytokines with disparate functions signal through JAK/STAT: γc-cytokines are involved in lymphocyte development and homeostasis; the gp-130 cytokine and the related dimeric cytokine family involve both pro- (IL-6, IL-12, IL-23) and anti-inflammatory (IL-27, IL-35) cytokines, the βc- and the hormone-like cytokines family involves critical growth factors for hematopoiesis like EPO, GM-CSF and G-CSF, and other hormones and growth factors. Important type II cytokines that use JAK/STAT pathways are the interferons (both type I and II) and the IL-10 family. The latter involves the anti-inflammatory IL-10, IL-20 critical for osteoclast formation and 22 involved in epithelial barriers integrity.The interaction between the activated receptors and homo- or heterodimers of JAK leads to the phosphorylation of the receptors, which, in turns allows the ligation phosphorylation and activation of STATs that enter the nucleus and regulate gene transcription.
Each cytokine receptor can activate more than one isoform of JAK, except for the γc chain of the receptor of γc -cytokines, which can only activate JAK3. Further complexity is added by the fact that each JAK isoform can activate different isoforms of STATs for downstream signaling. Tofacitinib is considered a pan-JAK inhibitor, active on JAK1, 2 and 3. Baricitinib is selective for JAK 1 and 2, peficitinib for JAK 1 and 3. Filgotinib and ABT-494 are JAK-1 selective agents, while decernotinib is a selective JAK3 inhibitor.
In the figure the cytokine pathways that are supposed to be predominantly blocked based on jakinibs selectivity are shown. Nevertheless, current data do not allow to determine whether those different selectivities do result in real differences in terms of efficacy or safety. Likewise, as each JAK isoform is involved in a number of cytokine pathways, it is not clear whether blockade of a single JAK isoform interferes with all these pathways and, if so, with the same potency. Hence, the patterns of (potentially) blocked cytokines can only partially explain the efficacy and tolerance profile of jakinibs.
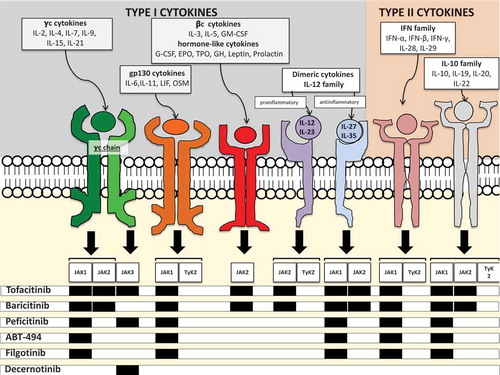
Figure 2. Simplified scheme of the three main scenarios of therapeutic intervention in RA: early cDMARDs naïve RA (a), methotrexate (or other cDMARDs) monotherapy therapeutic failure (b), cDMARDs combination or bDMARDs±cDMARDs therapeutic failure (c). The current or potential place of tofacitnib, and of newer jakinibs, based either on the FDA approval or on the recommendation of the scientific societies (yellow boxes) is shown. The clinical trials that support the positioning of the different jakinibs at the three different steps of therapeutic intervention are reported in blue boxes, with the corresponding reference in the text or clinical trial number. A) At present tofacitinib is not indicated in DMARDs naïve arthritis (dotted line). Several scientific societies recommend early intervention with a combination of cDMARDs or with bDMARDs in early, severe arthritis. The Oral-Start and the RA-Begin trials showed that tofacitinib and baricitinib monotherapies, respectively, were superior to methotrexate in controlling clinical activity in the setting of DMARDs naïve RA. This may support early therapeutic intervention with jakinibs in the future. B) The FDA approved tofacitinib in methotrexate insufficient responders both as sequential monotherapy and as add-on therapy to methotrexate or other cDMARDs. The 2015 ACR recommendations and the preliminary 2016 update of EULAR recommendations allow the use of tofacitinib in this setting, but with a suggestion to prioritize other approaches if possible. The Oral-Standard study showed that, in methotrexate insufficient responders, add-on therapy with tofacitinib was superior to placebo and numerically similar to adalimumab in efficacy. Both phase 2 and 3 studies support the use of other jakinibs as add-on or sequential monotherapy in this setting (blue box). C) In insufficient responders to cDMARDs combination, or to bDMARDs+/- cDMARDs, the use of tofacitinib is approved by the FDA and recommended both by the ACR and the EULAR [Citation25]. The clinical trials that support the use of the different jakinibs in this setting are reported in the blue box. ACR: American College of Rheumatology; bDMARDs biological disease-modifying drugs; cDMARDs: conventional disease-modifying drugs; EULAR European League Against Rheumatism; FDA: Food and Drug Administration.
![Figure 2. Simplified scheme of the three main scenarios of therapeutic intervention in RA: early cDMARDs naïve RA (a), methotrexate (or other cDMARDs) monotherapy therapeutic failure (b), cDMARDs combination or bDMARDs±cDMARDs therapeutic failure (c). The current or potential place of tofacitnib, and of newer jakinibs, based either on the FDA approval or on the recommendation of the scientific societies (yellow boxes) is shown. The clinical trials that support the positioning of the different jakinibs at the three different steps of therapeutic intervention are reported in blue boxes, with the corresponding reference in the text or clinical trial number. A) At present tofacitinib is not indicated in DMARDs naïve arthritis (dotted line). Several scientific societies recommend early intervention with a combination of cDMARDs or with bDMARDs in early, severe arthritis. The Oral-Start and the RA-Begin trials showed that tofacitinib and baricitinib monotherapies, respectively, were superior to methotrexate in controlling clinical activity in the setting of DMARDs naïve RA. This may support early therapeutic intervention with jakinibs in the future. B) The FDA approved tofacitinib in methotrexate insufficient responders both as sequential monotherapy and as add-on therapy to methotrexate or other cDMARDs. The 2015 ACR recommendations and the preliminary 2016 update of EULAR recommendations allow the use of tofacitinib in this setting, but with a suggestion to prioritize other approaches if possible. The Oral-Standard study showed that, in methotrexate insufficient responders, add-on therapy with tofacitinib was superior to placebo and numerically similar to adalimumab in efficacy. Both phase 2 and 3 studies support the use of other jakinibs as add-on or sequential monotherapy in this setting (blue box). C) In insufficient responders to cDMARDs combination, or to bDMARDs+/- cDMARDs, the use of tofacitinib is approved by the FDA and recommended both by the ACR and the EULAR [Citation25]. The clinical trials that support the use of the different jakinibs in this setting are reported in the blue box. ACR: American College of Rheumatology; bDMARDs biological disease-modifying drugs; cDMARDs: conventional disease-modifying drugs; EULAR European League Against Rheumatism; FDA: Food and Drug Administration.](/cms/asset/e0d8555c-1939-44dc-8326-7875fda1b6c0/ieid_a_1249565_f0002_oc.jpg)
