Figures & data
Figure 1. Patient flow.
PK: pharmacokinetic. aThe safety population consisted of subjects who received a single dose of study drug. bThe pharmacokinetic population consisted of all subjects who received a single dose of study drug, had at least one evaluable PK end point and were without protocol deviations or violations likely to impact the PK of the study drugs.
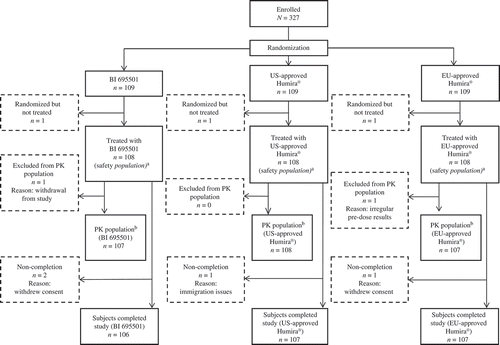
Table 1. Summary of PK parameters in healthy subjects after a single dose of BI 695501, US- or EU-approved adalimumab.
Figure 2. Arithmetic mean plasma concentration–time profiles in healthy subjects after a single dose of study drug for (a) BI 695501, US- and EU-approved Humira, (b) Forest plot presenting point estimate and 90% confidence intervals for primary PK parameters for BI 695, 501, US- and EU-approved Humira (bioequivalence was declared if the 90% confidence intervals were within prespecified acceptance ranges of 80–125%).
AUC0–inf, pred: AUC of the analyte in plasma over the time interval from zero extrapolated to infinity; AUC0–tz: AUC of the analyte in plasma over the time interval from time zero to the last measurable concentration; Cmax: maximum observed drug concentration in plasma; PK: pharmacokinetic.
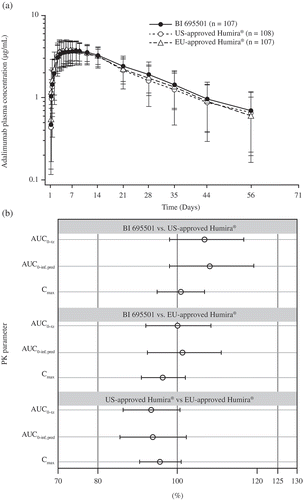
Figure 3. Development of ADAs in healthy subjects after a single dose of study drug at Days 28 and 71 for BI 695501, US- and EU-approved Humira. (a) Frequency of ADA-positive responses, (b) median ADA titer and (c) end-of-study titers for healthy subjects with ADA-positive responses. Median values are depicted by a line within the 25% and 75% percentile boxes; arithmetic mean = diamond shape; individual outliers = individual points; minimum and maximum values or 1.5× interquartile range = vertical lines out of the box plot. ADAs: antidrug antibodies.
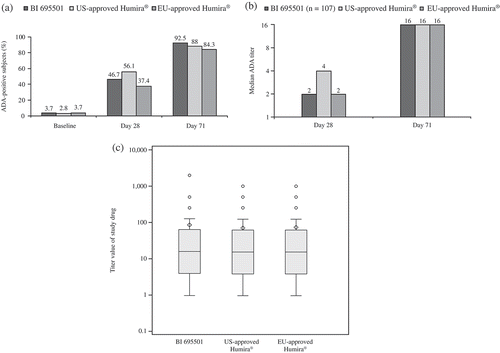
Figure 4. Neutralizing antibody development in healthy subjects after a single dose of study drug at baseline, Day 28 and Day 71 for BI 695501, US- and EU-approved Humira.
nAb: neutralizing antibody.
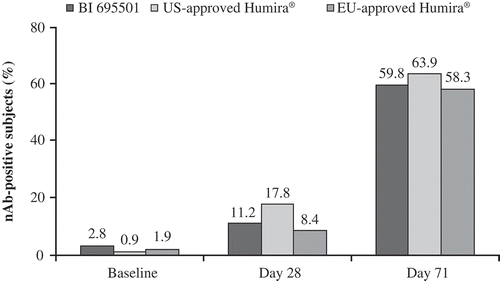
Figure 5. Impact of antidrug antibody titer (high [>16] vs. low [≤16]) on the exposure (AUC0–inf, pred) to the study drugs in healthy subjects after a single dose of BI 695501, US- or EU-approved Humira. Median values are depicted by a line within the 25% and 75% percentile boxes; arithmetic mean = diamond shape; individual outliers = individual points; minimum and maximum values or 1.5× interquartile range = vertical lines out of the box plot.
AUC0–inf, pred: AUC of the analyte in plasma over the time interval from zero extrapolated to infinity.
![Figure 5. Impact of antidrug antibody titer (high [>16] vs. low [≤16]) on the exposure (AUC0–inf, pred) to the study drugs in healthy subjects after a single dose of BI 695501, US- or EU-approved Humira. Median values are depicted by a line within the 25% and 75% percentile boxes; arithmetic mean = diamond shape; individual outliers = individual points; minimum and maximum values or 1.5× interquartile range = vertical lines out of the box plot.AUC0–inf, pred: AUC of the analyte in plasma over the time interval from zero extrapolated to infinity.](/cms/asset/b5b2d476-600d-4e0a-ac43-ffef8aef403d/ieid_a_1255724_f0005_b.gif)
Table 2. Summary of adverse events occurring in healthy subjects with one or more AEs, in any treatment group, after a single dose of BI 695501, US- or EU-approved Humira.
