Figures & data
Figure 1. Molecular pathways in DLBCL and possible therapeutic targets for small molecule inhibitors. A plenty of molecular pathways and their genomic alterations have been described in DLBCL, contributing to initiation, maintenance and progression of the disease. Activation or deregulation of these molecular pathways can impair different cell mechanisms such as epigenetic control, proliferation, differentiation, and apoptosis. Even if some of these molecular pathways are actionable through different small molecules, the coexistence of numerous molecular alterations in the same tumor hinder the progress of precision medicine in DLBCL. Despite these limitations, some new small molecules are under development and seem promising in this field
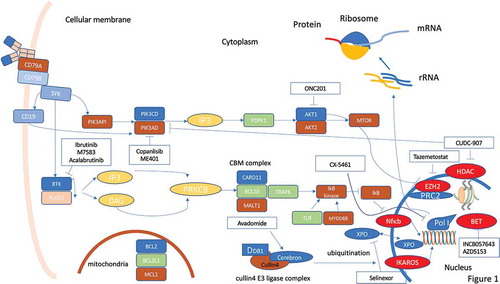
Table 1. Novel small molecules in DLBCL a
Figure 2. Druggable surface molecules in DLBCL. Surface antigens are the most reachable part of the cell, and monoclonal antibodies and cellular therapies targeting surface antigens represent an important therapeutic strategy in lymphomas. Several different antibodies, with different mechanisms of action, and CAR-T cells are under development for treating DLBCL, with the majority targeting CD19, CD20, CD22 due to the relative abundance of all these antigens on the cell surface. Other antigens such as CD79b (not shown) have been already used as target of ADCs (Polatuzumab-vedotin)
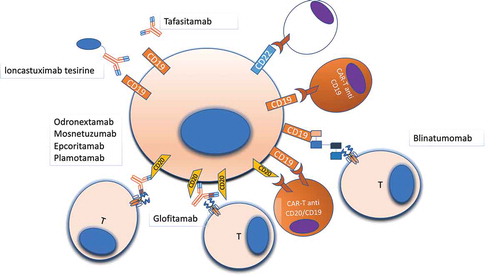
Table 2. Efficacy endpoints for different bispecific antibodies and CAR-T cells a
Table 3. Toxicity profile of bispecific antibodies and CAR-T cell treatments for DLBCLa
Figure 3. Schematic views of normal T cell receptor (TCR), chimeric antigen receptor (CAR), and antibody TCR. Panel A: TCR is composed of a binding outer domain that recognizes the antigen presented by antigen-presenting cells through MHC molecules. This interaction leads to the activation of different cellular pathways only in the presence of a costimulatory signaling through CD28/C4/C8/CD45. Panel B: CAR is composed of a scFV targeting antigen on cancer cells, a transmembrane linker and an inner portion containing both CDξδand the costimulatory CD28/4-1BB domain. This type of receptor is able to induce T-cell activation in an MHC-independent manner. Panel C: antibody TCR contains an intact γ/δ chain as transmembrane and intracellular domains, while the recognition domains is a Fab fragment targeting one specific antigen. Similar to CAR, also antibody TCR acts in an MHC-independent manner but retains the autoregulatory inhibition pathways similar to the normal TCR
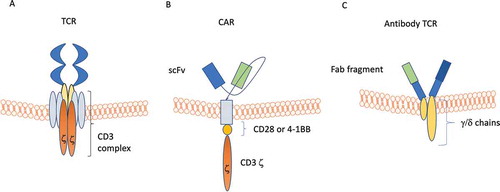
Figure 4. Mechanisms of evasion from anti-CD19 CAR-T cells. Panel A: in the case of loss of the target antigen, two different mechanisms have been described: i) internalization of the surface antigen (e.g. CD19); ii) amino-acidic modification of the CAR binding site. Panel B: in the case of cell lineage change, tumor cells can switch their phenotype to a different lineage, that constitutively does not express CD19. Panel C: epitope masking occurs when during lentiviral transfection a single leukemic or lymphoma cell is modified to express the CAR. Panel D: T-cell exhaustion may occur when the CAR-T cell population is chronically stimulated by the targeted antigen, leading to reduced T cell cytotoxic activity
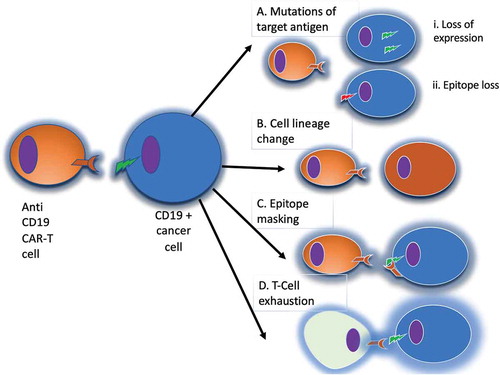
Table 4. New cellular technologies under development for relapsed/refractory DLBCL a.
