Figures & data
Table 1. Comparison of fezolinetant to first-generation NK3R antagonists osanetant and talnetant in rat. (A) Minimum effective dose correlates to brain receptor occupancy (e.g. unbound (‘free’) concentration of drug relative to receptor potency), (B) in vivo efficacy of NK3R antagonists measured by dose-dependent inhibition of plasma LH in castrate rat
Table 2. Receptor binding potency and selectivity at the human isoforms of the neurokinin receptor subtypes for fezolinetant, osanetant, and talnetant
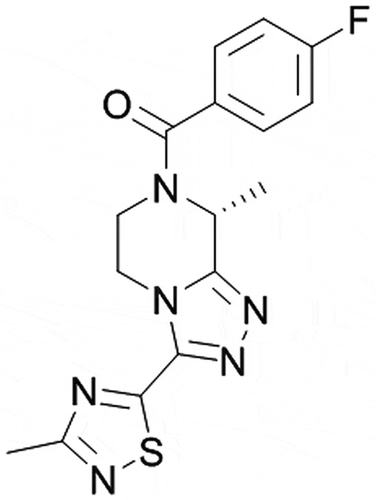
Figure 1. Mechanism of action of NK3R antagonists in VMS ERα: estrogen receptor alpha; FEZ: fezolinetant (NK3R antagonist); KNDy: Kisspeptin/Neurokinin B/Dynorphin; NKB: neurokinin B; NK3R: neurokinin-3 receptor; VMS: vasomotor symptoms
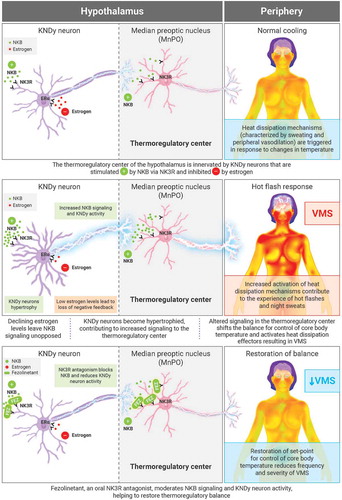
Table 3. Treatment-emergent adverse events in the phase IIb study
Figure 2. Effect of fezolinetant on VMS over time. (A) Daily total VMS score during week 4 and week 12. (B) Frequency of moderate/severe hot flashes during the duration of the study. (C) Moderate/severe VMS score during the duration of the study. Data are presented as mean ± 95% CI (mITT population, observed data). BID: twice daily; CI: confidence interval; FUP: follow-up; HF: hot flashes; mITT: modified intention to treat; VMS: vasomotor symptoms. Adapted from Depypere H, Timmerman D, Donders G, et al., Treatment of Menopausal Vasomotor Symptoms with Fezolinetant, a Neurokinin 3 Receptor Antagonist: A Phase 2a Trial, The Journal of Clinical Endocrinology & Metabolism 2019; 104 (12): 5893–5905 doi:10.1210/jc.2019-00677. Reprinted (and adapted) by permission of Oxford University Press on behalf of the Endocrine Society
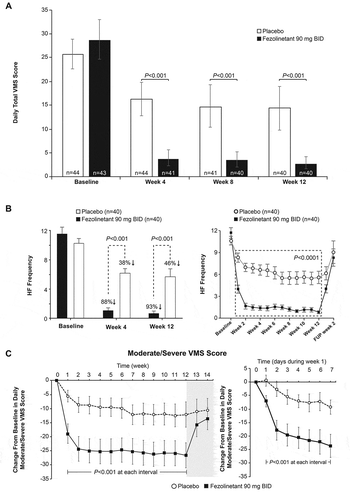
Figure 3. Effect of fezolinetant on quality of life measures. (A) LSEQ (does not include a total mean score that incorporates all four dimensions assessed). (B) HFRDIS. (C) GCS. (D) SDS. Data are presented as mean ± 95% CI (mITT population). BID; twice daily; CI: confidence interval; GCS: Greene Climacteric Scale; LSEQ: Leeds Sleep Evaluation Questionnaire; mITT: modified intention to treat; SDS: Sheehan Disability Scale. From Depypere H, Timmerman D, Donders G, et al., Treatment of Menopausal Vasomotor Symptoms with Fezolinetant, a Neurokinin 3 Receptor Antagonist: A Phase 2a Trial, The Journal of Clinical Endocrinology & Metabolism 2019; 104 (12): 5893–5905 doi:10.1210/jc.2019-00677. Reprinted by permission of Oxford University Press on behalf of the Endocrine Society
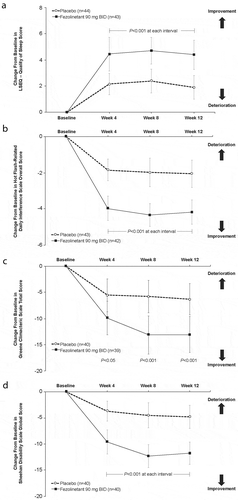
Table 4. Primary efficacy outcomes: frequency of moderate/severe VMS per 24 h, full analysis set
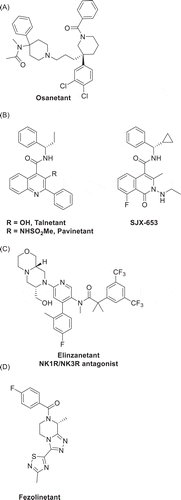
Figure 4. Structures of NK3R antagonists advanced into clinical development – (A) osanetant, (B) talnetant, pavinetant and SJX-653, (C) elinzanetant, (D) fezolinetant
Box 1. Drug summary box
Data availability statement
Researchers may request access to anonymized participant level data, trial level data, and protocols from Astellas sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.