Figures & data
Figure 1. Metabolism and key structural elements of bemnifosbuvir.
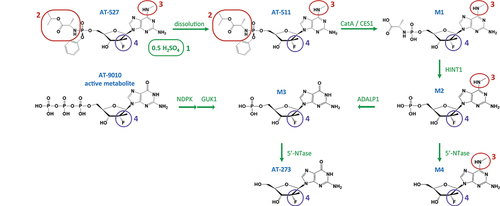
Figure 2. HCV RNA changes over 7 days of bemnifosbuvir monotherapy.
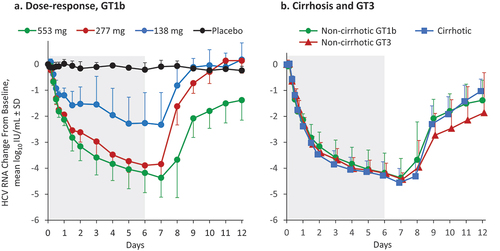
Figure 3. Pharmacokinetic/Pharmacodynamic analysis in patients with or without cirrhosis.
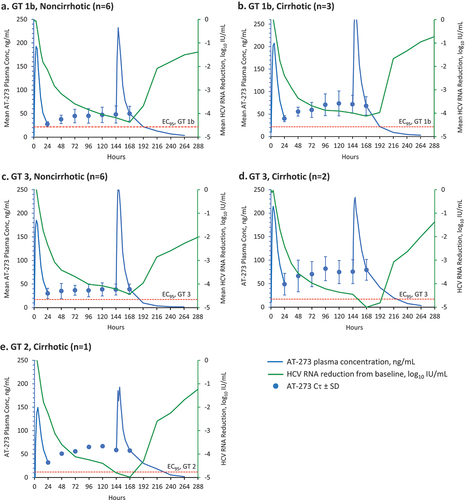
Figure 4. Emax model of bemnifosbuvir dose/anti-HCV response.
Figure 5. Viral kinetic simulation of bemnifosbuvir 550 mg QD as a single agent.
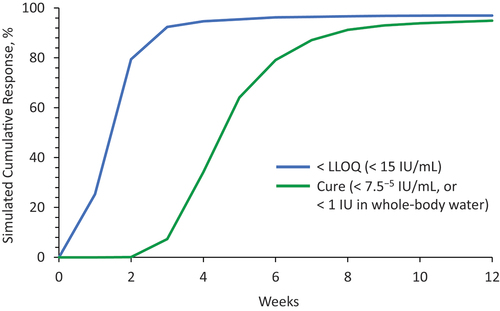
Table 1. Virologic outcomes with bemnifosbuvir + DCV.
