Figures & data
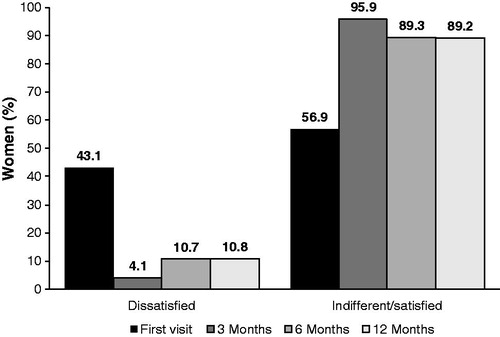
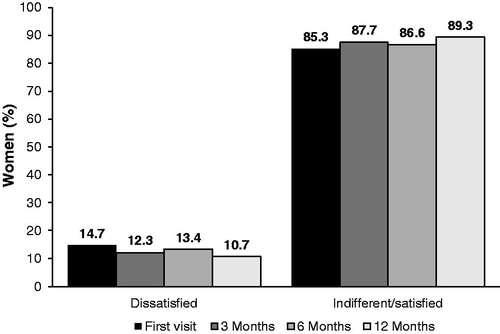
Figure 1. Participant disposition. Reasons for discontinuation of NOMAC/E2 included treatment-related AEs, poor compliance, dissatisfaction and decision to change contraceptive method. Discontinuation of NOMAC/E2 that was not treatment-related included no longer needed contraception, scheduled surgery and desire for pregnancy.
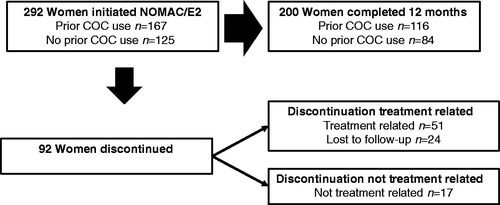
Table 1. Demographic and clinical characteristics of women receiving NOMAC/E2 (N = 298).
Table 2. Menstrual cycle and contraceptive method history of women receiving NOMAC/E2 (N = 298).
Figure 2. Probability of treatment continuation for 12 months among women receiving NOMAC/E2. The Kaplan–Meier discontinuation-free probability estimate from enrolment to day 365 was 73.7% (95% CI 68.0%, 78.5%). Censored women (n = 217) included treatment completers (n = 200) and women who discontinued for reasons not related to treatment (n = 17). Discontinuation events (n = 75) included women who discontinued treatment due to treatment-related AEs, poor compliance, dissatisfaction or decision to change contraceptive method (n = 51), and women lost to follow-up (n = 24).
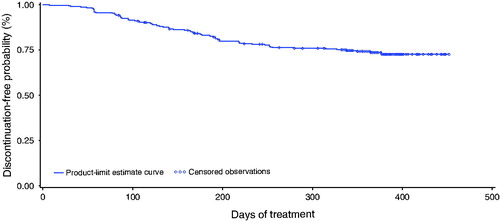
Table 3. Change from enrolment in menstrual cycle-related symptoms of women receiving NOMAC/E2.
Table 4. Treatment-related AEs reported by ≥2% of women receiving NOMAC/E2 (N = 292).