Figures & data
Box 1. Brands and characteristics of IUDs provided to EURAS-IUD study participants aged under 30.
Box 2. Factors and cost estimations as a consequence of IUD provision [Citation5–8]
Figure 1. EURAS-IUD study participants aged under 30 provided copper intrauterine contraception (IUDs). *Participants could report more than one problem.
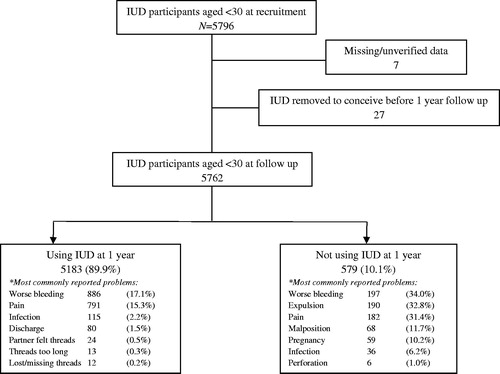
Table 1. Demographics and IUD types of EURAS-IUD participants aged under 30 at recruitment (N = 5796).
Table 2. IUD continuation or use status at 1 year follow up* [%, (n)].
Table 3. Unwanted effects including having to visit a HCP, expulsion and pregnancy at 1 year by IUD type.
Table 4. Unwanted effects and incidence of visiting a HCP in those participants still using the IUD at 1 year by IUD type.
Table 5. Unwanted effects including having to visit a HCP in those participants not using the IUD at 1 year by IUD type.
Table 6. Estimated cost consequences (£) at 1 year per 100 provisions by IUD type.
