Figures & data
Figure 1. Sex- and age- disaggregated data on deaths from COVID-19 in Europe. Data on mortality in males and females in Europe is shown in the graph. Data are derived from a specific dataset available at https://globalhealth5050.org/the-sex-gender-and-covid-19-project/the-data-tracker [Citation6]. As a compromise between accurate age- and sex-stratification and sufficient data density, we included in the analyses only European countries with sex-disaggregated data about number of deaths per 100,000 population divided into 10-year age groups. The final selection included data from: Czech Republic, Denmark, England, France, Hungary, Israel, Italy, Poland, Portugal, Spain, Switzerland, Wales. The mean about separate males and females’ mortality of all the countries selected was done. Then, the ratio between males and females’ mortality per 100,000 population was calculated for every 10-year age group.
![Figure 1. Sex- and age- disaggregated data on deaths from COVID-19 in Europe. Data on mortality in males and females in Europe is shown in the graph. Data are derived from a specific dataset available at https://globalhealth5050.org/the-sex-gender-and-covid-19-project/the-data-tracker [Citation6]. As a compromise between accurate age- and sex-stratification and sufficient data density, we included in the analyses only European countries with sex-disaggregated data about number of deaths per 100,000 population divided into 10-year age groups. The final selection included data from: Czech Republic, Denmark, England, France, Hungary, Israel, Italy, Poland, Portugal, Spain, Switzerland, Wales. The mean about separate males and females’ mortality of all the countries selected was done. Then, the ratio between males and females’ mortality per 100,000 population was calculated for every 10-year age group.](/cms/asset/dc79bc21-2b0a-4575-985a-26e980f0a598/iejc_a_2000959_f0001_b.jpg)
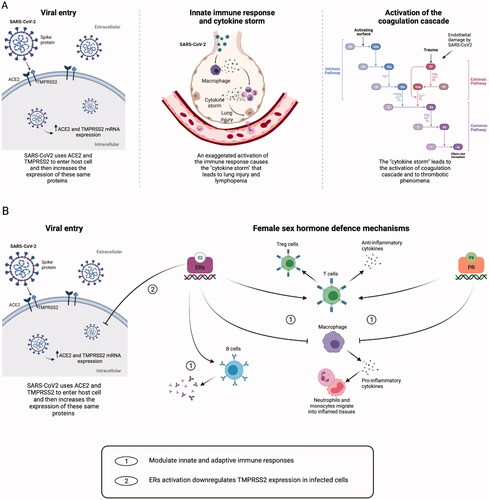
Figure 2. Mechanisms of tissue damage by SARS-CoV2 and sex-hormone defence mechanisms. (A) Severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) spike proteins recognise the host cell via the angiotensin-converting enzyme 2 (ACE2) and the serine protease Transmembrane Protease Serine 2 (TMPRSS2), leading to virus entry. SARS-CoV2 infection can enhance the ACE2 and TMPRSS2 messenger ribonucleic acid (mRNA) gene expression. Immune cells infiltrate the lungs, causing hyperactivation of monocytes and macrophages, with production of pro-inflammatory cytokines, tissue necrosis factor-β (TNF-β) and chemokines. The exaggerated activation of the immune response leads to the so-called ‘cytokine storm’, and this situation causes lymphopenia and lung injury. Tissue damage ultimately leads to the activation of the extrinsic pathway of the coagulation cascade. (B) Both oestradiol (E2) and progesterone (P4) can modulate innate and adaptive immune response by binding their nuclear receptors. Both hormones can inhibit macrophage activation, thus reducing the secretion of pro-inflammatory cytokines and the migration of neutrophils and monocytes into inflamed tissues and can stimulate the production of anti-inflammatory cytokines and the expansion of T regulatory (Treg) cells. Moreover, E2 can enhance the production of antibodies by B cells, and it inhibits the increasing expression of TMPRSS2 in infected cells, possibly reducing the virus content inside the cells. Created with BioRender.com. ERs: oestrogen receptors; PR: progesterone receptor; TF: tissue factor.