Figures & data
Table 1. Demographic characteristics of participants who completed the MDQ at baseline and at end of treatment in the Europe/russia phase 3 trial with E4/DRSP (N = 1398).
Table 2. Mean baseline, EoT and change from baseline for menstrual and premenstrual MDQ t-scores in the Europe/russia phase 3 trial with E4/DRSP.
Figure 1. Mean baseline and EoT premenstrual [A] and menstrual [B] MDQ t-scores for starters and switchers in the Europe/Russia phase 3 trial with E4/DRSP.
![Figure 1. Mean baseline and EoT premenstrual [A] and menstrual [B] MDQ t-scores for starters and switchers in the Europe/Russia phase 3 trial with E4/DRSP.](/cms/asset/962865aa-cdb8-474f-a782-fae9667a28e6/iejc_a_2359117_f0001_c.jpg)
Table 3. Mean baseline, EoT and change from baseline for premenstrual and menstrual MDQ t-scores in the Europe/russia phase 3 trial with E4/DRSP - starters and switchers.
Figure 2. Shift analysis for premenstrual and menstrual symptom scores for Painful or tender Breasts in the physical domain ‘Water Retention’ for starters and switchers who completed the MDQ at baseline and at end of treatment in the Europe/Russia phase 3 trial with E4/DRSP.
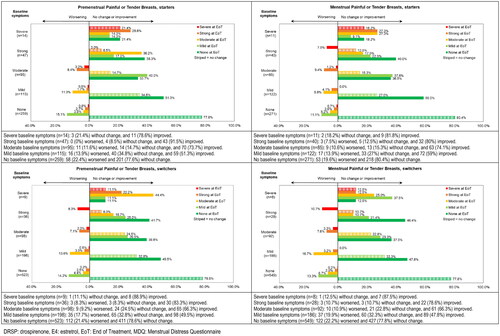
Figure 3. Shift analysis for premenstrual and menstrual symptom scores for Mood Swings in the emotional domain ‘Negative Affect’ for starters and switchers who completed the MDQ at baseline and at end of treatment in the Europe/Russia phase 3 trial with E4/DRSP.
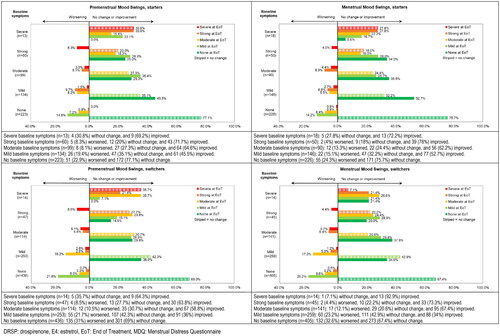
Figure 4. Shift analysis for premenstrual and menstrual symptom scores for Irritability in the emotional domain ‘Negative Affect’ for starters and switchers who completed the MDQ at baseline and at end of treatment in the Europe/Russia phase 3 trial with E4/DRSP.
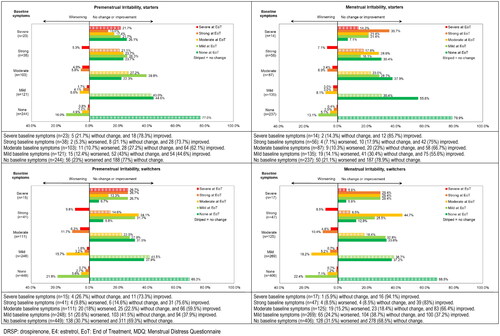
Table 4. Overview of shift analysis with % of participants remaining stable, improving and worsening and % of participants with severe or strong symptoms at baseline for premenstrual and menstrual symptoms in the Europe/Russia phase 3 trial with E4/DRSP - starters and switchers.
