Figures & data
Figure 1. Disposition of subjects. 1000 subjects from a non-selected population were recruited across Germany between January 2005 and December 2006. In total 870 subjects were analyzed.
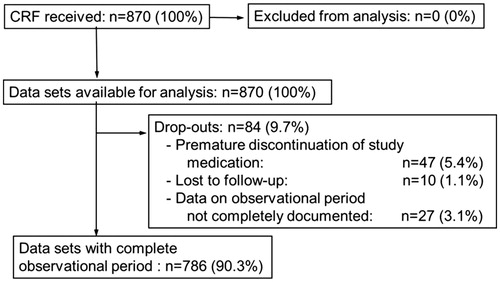
Figure 2. Assessment of the efficacy of Nebido at each visit. Over the time course of the observational period, over 90% of respondents described the efficacy of Nebido as very good or good with an overall increase of very good responses over the same period. Very few respondents described Nebido's efficacy as poor.
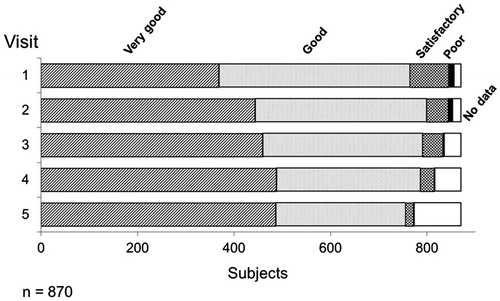
Table 1. Average serum testosterone levels measured at certain visits after treatment with Nebido.
Figure 3. Assessment of the tolerability of Nebido at each visit. Over the time course of the observational period, over 90% of respondents described the tolerability of Nebido as very good or good with an overall increase of very good responses over the same period. Very few respondents described Nebido's tolerability as poor.
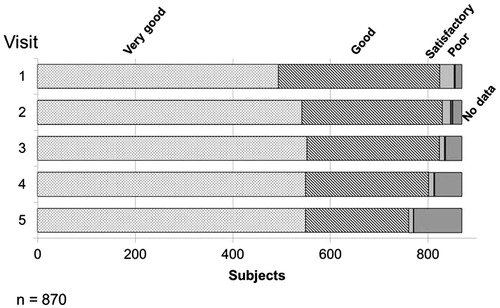
Figure 4. Changes of mean body weight with 1 year of Nebido treatment. The effect on body weight was differential with underweight and normal weight patients showing modest weight gain whilst overweight and obese subjects experienced weight loss.
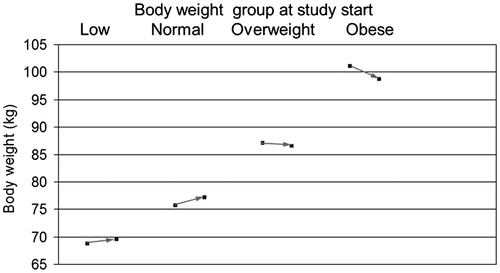
Table 2. Changes in average body weight, BMI, and waist girth during 1 year of treatment with Nebido.
Table 3. Body weight – gain and loss within 1 year on Nebido.